Controlling Factors of the Population Dynamics of Two Dominant Bivalves of the Macro-benthic Community on the Sandy Tidal Flats
Oceanography & Fisheries Open Access Journal Juniper Publishers
Abstract
The edible short-neck clam, Ruditapes philipinnarum, is one of the most dominant species in the macro-benthic community on the sandy tidal flats that face Ariake Bay in Kumamoto Prefecture, western Japan. Until the 1970s, over 40,000 tons of the clams were collected per year on the tidal flats. However, the dense patches disappeared, and the clam-harvesting fishery has suffered from extremely poor catches of less than 500 tons per year over the past three decades. We conducted environmental assessments of the sediment and did quantitative surveys of the macro-benthic community on Midori River Tidal Flats located in Kumamoto between April 2017 and April 2019 and tried to find the reasons why the clam population markedly declined. Asian mussels (Arcuatula senhousia) and short-neck clams predominated the macro-benthic community on the tidal flats. However, the former species was subject to heavy predation by ducks that visited the tidal flats during the winter, while the latter one suffered from predation by rays during the warm seasons. Although Asian mussels also affected the occurrence of short-neck clams by formation of muddy carpets on the sediment, they were accidentally destroyed due to strong wind and waves caused by a typhoon.
Keywords: Asian mussels; Muddy carpet; Population dynamics; Predation; Short-neck clams; Tidal flats
Introduction
Introduction
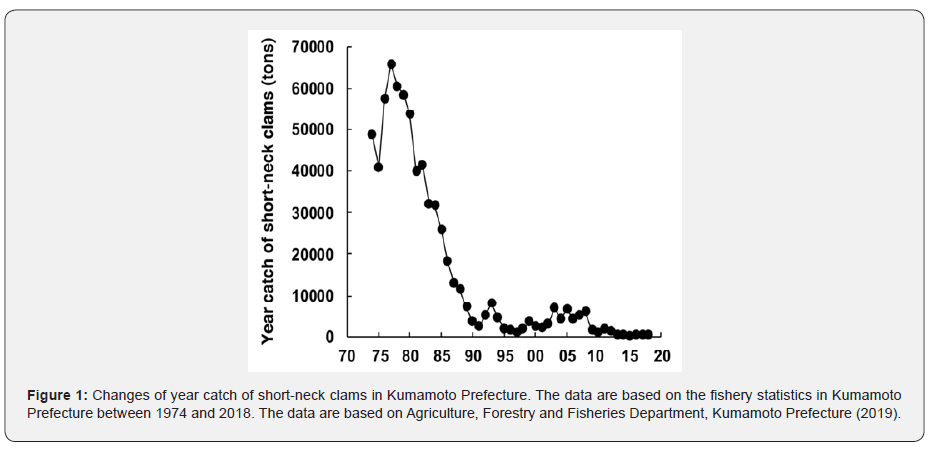
Approximately 20,000 ha of tidal flats still remain along the coast of Ariake Bay, Kyushu, western Japan. There, innumerable blue spotted mud hoppers (Boleophthalmus pectinirostris), fiddler crabs (Uca arcuata), crabs (Macrophthalmus japonica), etc., feed on benthic diatoms thickly covered on the surface of the muddy bottom [1], and various suspension feeding clams including Ruditapes philippinarum (short-neck clams), Meretrix lusoria (Japanese hard clams) and Mactra venerformis (surf clams), etc. occur densely on the sandy bottom[2,3]. In the coast of Kumamoto Prefecture that faces Ariake Bay, most of the tidal flats are sandy, and have been used as fishery grounds for harvesting clams. Until the late 1970s, 40,000 to 65,000 tons of short-neck clams were collected on the tidal flats per year, which accounted for approximately half of the national annual catch of the clams in those days. However, it dramatically decreased in the 1980s, and declined to less than 500 tons in 1995, although the total area of the tidal flats was kept intact and the clam harvesting activities had been strictly controlled by fisherman’s associations to avoid over-fishing [4].
Nevertheless, until today, it has not shown significant recovery [5,6] (Figure 1). This dramatic decline of the clam catch indicates that the abundant primary production by microphytobenthos and phytoplankton on the tidal flats, which are available for the clam as main diets [7], is not reflected as the secondary production of the clams in the past four decades. Previous studies dealing with the occurrence of the clam on the tidal flats in Kumamoto Prefecture have found various causes of the collapse of clam harvesting fishery on the sandy tidal flats, which involve environmental disturbances due to the occurrence of extremely low salinity caused by a large amount of freshwater discharged from the river and deposition of mud transported from the upper reaches of the river during the rainy season [8], the elevation of manganese content of the sediment to intolerable levels for juveniles just after the settlement [9,10], the decrease of planktonic larvae produced during the breeding season [11], and the predation by moon snails (Glossaulax didyma) and rays (Aetobatus flagellum and Hemitrygon akajei) [12,13].
Recently, the sandy tidal flats have been thickly covered by the muddy carpets created by Asian mussels (Arcuatula (Musculista) senhousia) across the Japanese coast [14]. Those in Kumamoto Prefecture are not exceptional [15-17]. They provide new micro-habitats that enable various infaunal animals occur in them, while the sediment under them tends to fall to extremely reduced conditions as original dominant members of suspension-feeding bivalves, including short-neck clams, duck clams (Mactra quadrangularis) and razor clams (Solen strictus), etc. cannot physiologically adapt [14,15,18]. This species has invaded to the lagoons in various countries including New Zeeland [19], Australia [20,21], Europe [22-24], North America [25,26] etc., and given negative impacts on the domestic benthic ecosystem, as established dense patches with the muddy carpets [27]. The latest studies on the impact of the formation of muddy carpets by the Asian mussel on the sandy tidal flats revealed that it not only created intolerable environmental conditions for suspension-feeding clams in the sediment, but also created diet-short conditions for them by promoting the deposition of organic particles suspended in the water inside the muddy carpets [17,28].
This behavior of Asian mussels appears to be a kind of “indirect exploitative type of interference” to co-occurring other species as monopolize the food resources before the competitive species utilize them [29]. Thus, Asian mussels that create muddy carpets have potentially a strong capacity for occupying surface space on the sediment of the soft bottom just like Mediterranean mussels tend to monopolize the rock surface on the rocky shore [30,31]. The invasion process by Asian mussel on the tidal flats starts by mass settlement of planktonic larvae just after the breeding season in summer. Tsutsumi et al. [15] described the invasion process as it could establish dense patches around 5,000 ind. m-2 and 2,000 gww m-2 creating muddy carpets within several months once its mass recruitment occurred, and finally the position of the predominant species of the macro-benthic community was totally replaced with short-neck clams. None of the animals could affect the population fluctuations of the Asian mussels in this study conducted in 2008 to 2009 as long as its dense patches were mechanically destroyed by the experiment that artificially turned over the sediment with a power shovel.
In the previous studies, as predatory animals on Asian mussels in Japan, Yamamuro et al. [32] reported that several species of diving ducks that visited a lagoon for wintering favored to feed on Asian mussels and gave a marked impact on abundance during the winter. Ito [33] also found the shells of Asian mussels from the stomach contents of a specimen of dabbling duck, Anas platyrhynchos, captured beside the red algae, nori, cultivation farms set on the tidal flats. Nori has been cultivated extensively on the tidal flats and their offshore areas across the Japanese coast during the winter, and recently suffered from serious feeding damage by ducks [33-35]. In this study, we conducted the environmental assessments of the sediment and quantitative surveys of macro-benthic community on Midori River Tidal Flats, which has been one of the major sandy tidal flats that face Ariake Bay in Kumamoto Prefecture, between April 2017 and April 2019.
Here, short-neck clam-harvesting fishery activity is still carried on throughout the year, while nori cultivation farms are also set extensively during the winter. Short-neck clams originally predominated the macro-benthic community on the tidal flats, but mass-settlement of Asian mussels has recently occurred and followed the formation of muddy carpets [15]. In this paper, we report the results of the environmental assessments of the sediment and quantitative surveys of macro-benthic community on the tidal flats, analyze the mechanisms of the seasonal fluctuations of the populations of the two competitive dominant bivalves in the macro-benthic community, and discuss what factors mainly controlled their population dynamics and what we should do to re-establish dense patches of short-neck clams to recover its harvesting fishery on the tidal flats as much as actively done in the 1970s.
Materials and Methods
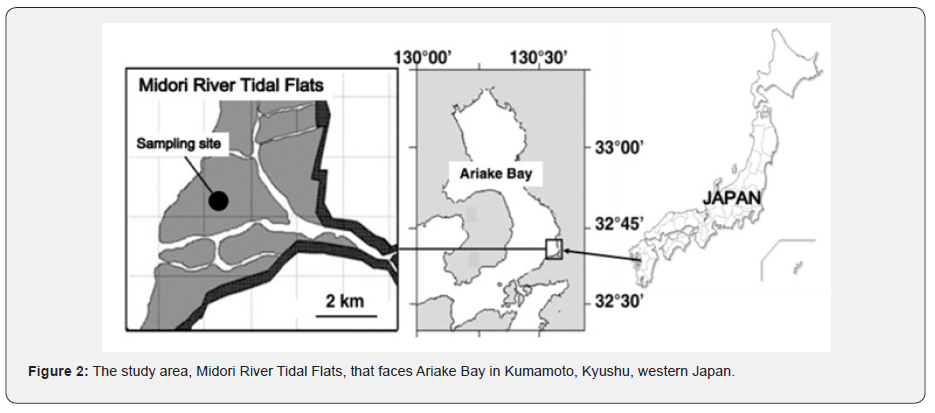
The Midori River Tidal Flats is located at the river mouth of Midori River, Kumamoto, Kyushu, western Japan. It extends 5 km toward the offshore with the area of approximately 2,200 ha during the low tide in spring tide. We established a sampling site at the lower part on the tidal flats (N 32° 43′ 35.3″,E 133° 41′ 11.5″, Figure 2), where clam-harvesting fishery activity was actively carried on until the 1980s. The sampling site appears above the water when the tide level has descended to less than 40 cm.
We conducted field surveys at the sampling site during the low tide in spring tide monthly or bimonthly (15 times in total) between April 2017 and April 2019. At each sampling occasion, we collected a sediment sample up to the depth of 5 cm with a core sampler (5 cm x 5 cm x 5 cm), which was kept in a plastic bag. We also collected ten sediment samples for quantitative sampling of macro-benthic animals with a core sampler (10 cm x 10 cm x 5 cm). Each sample was sieved with a 1 mm opening mesh, and the residues retained on the mesh were kept in a plastic bag with 10 % formalin solution and a dye, Rose Bengal.
At the laboratory, the particle size composition of the sediment was determined with the sediment sample by wet-sieving method. The samples fixed with formalin solution were washed and sieved on a 1 mm opening mesh again. All of the macro-benthic animals were sorted from the residues on the mesh and identified as to its species. The total number and the wet weight of each species were confirmed. The shell lengths of the specimens of Asian mussels and short-neck clams were measured with a digital caliper.
The daily changes ratios of density (DCRD) and biomass (DCRB) of Asian mussels and short-neck clams were calculated with the following equations between two successive sampling occasions.
DCRD = (Densityi - Densityi-1)/(Di - Di-1)
DCRB = (Biomassi - Biomassi-1)/(Di - Di-1)
Densityi : Density at the sampling occasion I; Biomassi : Biomass at the sampling occasion I; Di : The number of days that passed from the survey start, 27 April 2017.
The shell length frequency distributions of the populations of Asian mussels and short-neck clams were drawn with the data of the shell length of the specimens collected at each sampling occasion, and they were treated with moving average method once by calculating the mean frequency at each shell length class every mm with shorter and larger classes to get a smoother shell length frequency distribution. In general, the shell length frequency distribution of the population is polymodal, since it is made up of a number of monomodal ones of the cohorts. It can be divided into these with a graphic method modified from Harding [36] for cohort analysis, which was computer-programmed as PROGEAN (PROgram for Generation Analysis) for a personal computer, NEC PC9801 series [37]. The shell length frequency distributions of the populations of Asian mussels and short-neck clams were divided to those of the cohorts, using PROGEAN Ver. 4.0.
Results
Seasonal Changes in Grain Size Composition of the Sediment
Figure 3 indicates seasonal changes of the grain size composition of the sediment at the sampling site on the tidal flats between April 2017 and April 2019. The mud content of the sediment (the weight composition of fine particles of less than 63 µm in diameter) was kept above 9.8 % between April and November 2017, and the highest one, 41.3 %, was noted in May. This high mud content of the sediment was caused by the bio-deposition of fine particles suspended in the water by Asian mussels. The sediment surface was covered by muddy carpets created by this species (Figure 4a).
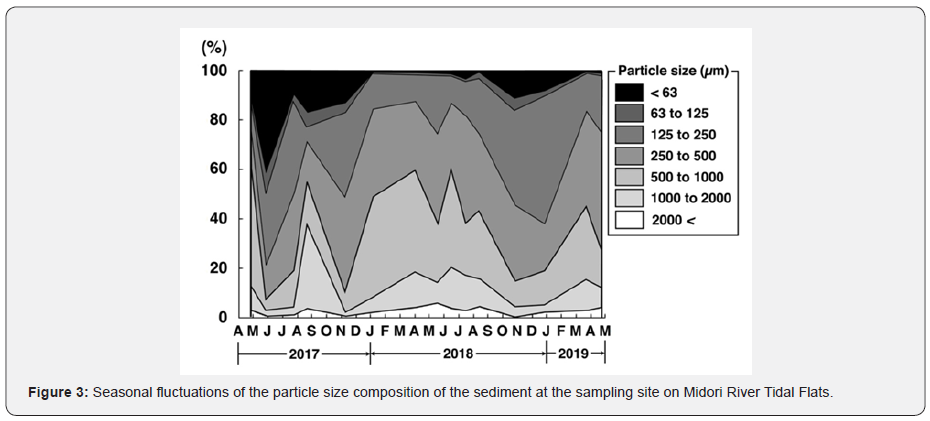

The mud content of the sediment rapidly decreased to 9.8 % in July, once recovered to 17.3 % in August, but decreased from 13.5 % in November 2017 to 1.0 % in January 2018 again. Since then, it fluctuated within a narrow range between 0.9 and 3.5 % until August 2018. In this period, 77.4 to 90.1 % of the sediment was made up of three components of sand (coarse sand: 500 to 1,000 µm, medium sand: 250 to 500 µm, and fine sand: 125 to 250 µm in diameter) (Figure 4b), which is the original conditions of the sediment as sandy tidal flats without the muddy carpets [4]. In October 2018, the sediment surface was temporarily covered by the muddy carpets again, and the mud content of the sediment increased to 11.5 % (Figure 4c). However, it returned to the sandy sediment with the mud content of less than 1 % by March 2019 (Figure 4 d).
Seasonal Changes of Density of Macro-Benthic Community
Figure 5a indicates seasonal fluctuations of the density of the macro-benthic community at the sampling site between April 2017 and April 2019. The community involved two exclusively dominant species, Asian mussels (A. senhousia) and short-neck clams (R. philippinarum), which occupied 48.1 % and 34.7 % of all of the specimens collected in this study, respectively. The remaining 17.2 % of the community was made up of Reticunassa festiva (snails), amphipods, polychaetes, etc. Asian mussels established dense patches of 11,290 ind. m-2 in May 2017, but the density suddenly decreased to about its half, 5,670 ind. m-2, in July. The density remained 3,670 ind. m-2 in August and 5,190 ind. m-2 in November but decreased to only 180 ind. m-2 by January 2018 and remained in low densities of 30 to 1,200 ind. m-2 until June 2018.
This species explosively increased its density from June and established extremely high-density patches of 92,140 ind. m-2 only within a couple of month and kept the dense patches of 25,610 ind. m-2 until October. However, the dense patches collapsed during the late autumn and winter again as did in the last year and returned to the extremely low density of only 40 ind. m-2 by March 2019. Short-neck clams showed more stable fluctuations of the density between 1,320 and 4,310 ind. m-2 between April 2017 and January 2018. The density increased to 39,340 ind. m-2 in May, but it also decreased to only 300 ind. m-2 by October, and the low-density patches of 200 to 410 ind. m-2 remained until April 2019. The total density of other macro-benthic animals gently fluctuated between 720 and 6,020 ind. m-2 throughout the period of this study except 17,960 ind. m-2 in April 2018.
Figure 5b indicates seasonal fluctuations of DCRD values of two exclusive dominant bivalves between May 2017 and April 2019. They further clearly show the characteristics of the seasonal fluctuations of the densities of these two species. In Asian mussels, they were characterized by an explosive increase between July and August 2018 (+3,108 ind. m-2 d-1), small scales of decreases between May and August 2017 (-96.9 ind. m-2 d-1 in July and -74.1 ind. m-2 d-1 in August) and between November 2017 and January 2018 (-84.9 ind. m-2 d-1 in January) and a collapse of dense patches between August and December 2018 (-887 ind. m-2 d-1 in October and -374 ind. m-2 d-1 in December). In short-neck clams, they were characterized by the stable fluctuations of the density between April 2017 and January 2018 (-30.3 to +27.1 ind. m-2 d-1) and followed by a rapid increase between January and May 2018 (+383 ind. m-2 d-1 in April, +112 ind. m-2 d-1 in May) and a collapse of the dense patches between May and August 2018 (-798 to -100 ind. m-2 d-1).
Seasonal Changes of Biomass of Macro-Benthic Communities
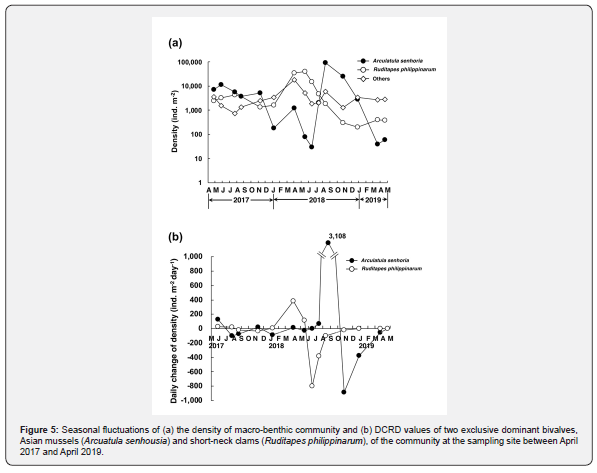
Figure 6a indicates seasonal fluctuations of the biomass of the macro-benthic community expressed by wet weight at the sampling site between April 2017 and April 2019. In biomass, Asian mussels and short-neck clams also predominated the macro-benthic community and occupied 47.1 % and 42.3 % of the total biomass of the specimens collected in this study, respectively. The remaining 10.6 % was made up of R. festiva, amphipods, polychaetes, etc. as the same manners with the density. The fluctuation patterns of the
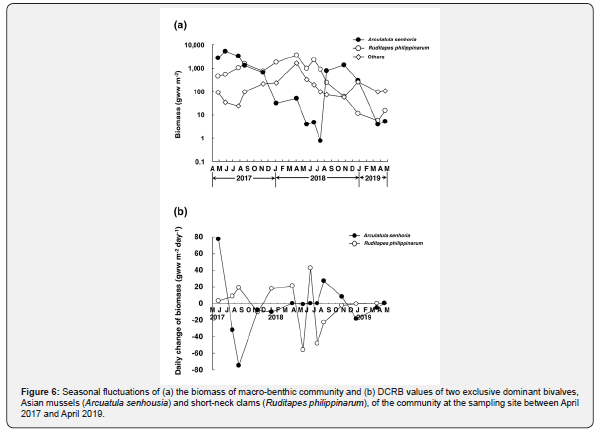
biomass of the two dominant species roughly coincided with those of their densities. Asian mussels established dense patches of 5,186 gww m-2 in May 2017, but the biomass decreased to only 0.8 gww m-2 by July 2018. It rapidly increased to 1,406 gww m-2 by October once, following the explosive increase of the density (Figure 5a), but decreased to only 3.9 gww m-2 by March 2019 again. In contrast, the biomass of short-neck clams gradually increased from 452 gww m-2 in April 2017 to 3,572 gww m-2 in April 2018. However, it also decreased to 993 gww m-2 in May and decreased to only 5.8 gww m-2 by March 2019, although it once soon recovered to 2,274 gww m-2 in June 2018.
Figure 6b indicates seasonal fluctuations of DCRB values of the two exclusive dominant species at the sampling site between May 2017 and April 2019. The changes of DCRB values clearly revealed the occurrence of big changes of their biomass further. In Asian mussels, the fastest decline of biomass occurred between May and August 2017 (-32.2 gww m-2 d-1 in July, -74.9 gww m-2 d-1 in August), and it also suffered from the decline of the biomass during the autumn and winter (-8.2 gww m-2 d-1 in November 2018 and -10.5 gww m-2 d-1 in January 2018; -18.2 gww m-2 d-1 December 2018 and -5.4 gww m-2 d-1 in March 2019). The biomass increased between July and October 2018 (+27.0 gww m-2 d-1 in August, +8.3 gww m-2 d-1 in October 2018), following the explosive increase of the density (Figure 5a). In short-neck clams, the DCRB value had ranged between +3.0 and +21.0 gww m-2 d-1 until April 2018 except -10.1 gww m-2 d-1 in November 2017. It decreased to -56.1 gww m-2 d-1 in May 2018, once returned to positive, 42.7 gww m-2 d-1, in June, but it became negative again -48.4 gww m-2 d-1 in July and -23.1 gww m-2 d-1 in August.
Analysis of Frequency Distribution of Shell Length in the Population
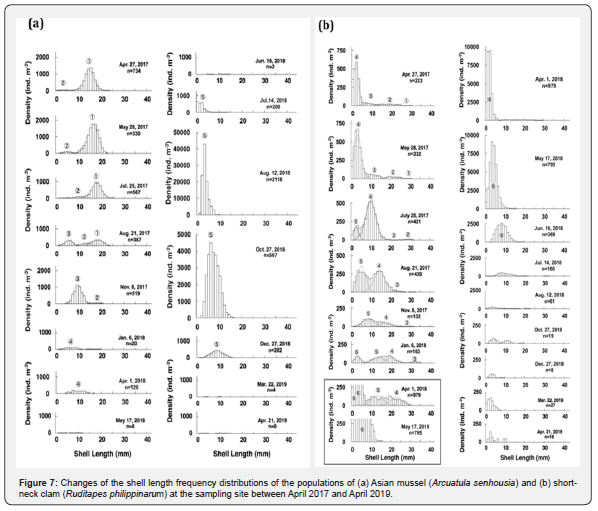
The population dynamics of the two dominant bivalves, Asian mussels, and short-neck clams, in the macro-benthic community between April 2017 and April 2019 are characterized as mentioned below, based on the results of the analysis of seasonal fluctuations of the densities and biomass. We checked each of the characteristics of the population dynamics of these two species with the changes of their shell length frequency distributions (Asian mussels in Figure 7a, short-neck clams in Figure 7b) to clarify the mechanisms that caused each of the characteristic events.
Asian Mussels
Rapid decline of the biomass between 28 May and 21 August 2017
The shell length frequency distribution of the population on 21 August 2017 indicates that the population was made up of three cohorts with the shell length of 18.6±2.1 mm (mean ± S.D.)(Cohort 1), 13.4 ± 1.9 mm (Cohort 2) and 5.7 ± 1.7 mm (Cohort 3). The rapid decline of the biomass of the population (from 5,186 gww m-2 in May to 1,297 gww m-2 in August) was responsible for the marked decrease of density of Cohort 1 (from 10,819 ind. m-2 in May to 1,808 ind. m-2 in August) .
Rapid decline of the density and biomass between 8 November 2017 and 6 January 2018
The newly recruited Cohort 3 to the population in August grew up to the shell length of 9.9 ± 1.9 mm with the density of 5,054 ind. m-2 on 8 November 2017, but most members disappeared on 6 January 2018. This event indicates that Cohort 2 was subject to a strong mortality factor during this period.
Explosive population growth between 14 July and 27 October 2018
Mass recruitment by Cohort 5 to the population initiated on 14 July 2018. 42,950 ind. m-2 of the mode density of its shell length frequency distribution was recorded at the shell length class of 3 to 4 mm on 12 August, and it formed dense patches with the density of 25,610 ind. m-2 and biomass of 1,406 gww m-2 on 27 October.
Collapse of Cohort 5 between 27 October 2018 and 22 March 2019
The population was made up of only a single cohort, Cohort 5, in October 2018. As shown in the shell length frequency distribution of the population, it rapidly declined and almost disappeared by 22 March 2019, in the same manners as the rapid decline of Cohort 3 between 8 November 2017 and 6 January 2018. Thus, most members of the cohort, which were recruited to the population in the breeding season in early summer, suffered from a strong mortality factor during the late autumn and winter.
Stable fluctuations of the density and gradual increase of biomass between 27 April 2017 and 6 January 2018
The shell length frequency distribution of the population on 28 May 2017 indicates that the population was made up of four cohorts, which had the shell lengths of 29.2 ± 0.9 mm (mean ± S.D.)(Cohort 1), 21.4 ± 2.6 mm (Cohort 2), 11.8 ± 2.0 mm (Cohort 3) and 3.5 ± 1.9 mm (Cohort 4), respectively. Short-neck clams have two breeding seasons (spring and late autumn) in Kumamoto in a year (Kumamoto Prefecture Fisheries Research Center, 2006). The recruitment of a new cohort to the population was clearly recognized in the shell length frequency distribution of the population in each of two breeding seasons in 2017. Consequently, the clam population consisted of four cohorts, which had the shell lengths of 31.4 ± 2.3 mm (Cohort 3), 20.6 ± 2.2 mm (Cohort 4), 13.6 ± 2.9 mm (Cohort 5) and 3.1 ± 1.5 mm (Cohort 6) on 6 January 2018. Thus, the growth and survival processes of these cohorts could be traced along the changes of the shell length frequency distribution of the population during this period. It indicates that the clam population was not subject to strong mortality factors.
Rapid increase of density between 6 January and 17 May 2018
Cohort 6 first appeared in the shell length frequency distribution of the population on 6 January 2018, and it expanded to a mass recruitment with peak densities of around 9,100 ind. m-2 at the shell length classes of less than 4 mm on 17 May 2018. Actually, the members of this cohort have already settled on the sediment during the autumn breeding season in 2017, but they were too small to retain on the sieve with 1 mm opening mesh used for sampling. As they grew up to the shell sizes that retained on the sieve in the spring, the density rapidly increased.
Rapid decline of the biomass between 1 April and 17 May 2018
The density of the population slightly increased from 34,179 ind. m-2 to 39,340 ind. m-2 in this period (Figure 5a), while its biomass markedly decreased from 3,752 gww m-2 to 993 gww m-2 (Figure 6a) and the largest negative DCRB, -56.1 gww m-2 d-1, was noted on 17 May 2018 (Figure 6b). These contradictory changes between the density and biomass of the population were responsible for the selective elimination of Cohort 4 and Cohort 5 with the shell length of more than 17 mm from the population (see inside the frame in Figure 7b). However, this loss was compensated by the rapid increase of Cohort 6 in number.
A sign of the drastic change of the population between 17 May and 16 June 2018
The density of the population decreased to less than half in this period (15,390 ind. m-2, Figure 5a, but its biomass, nevertheless, temporarily recovered to more than double (2,274 gww m-2, Figure 6a). The fast individual growth of Cohort 6 (from 4.2±2.2 mm to 8.5±2.9 mm in shell length) due to warm conditions contributed to the increase of biomass of the population.
Collapse of the population between 16 June and 27 October 2018
The population that consisted of only Cohort 6 collapsed, and its density and biomass decreased to only 300 ind. m-2 (Figure 5a) and 61.1 gww m-2 (Figure 6a) in this period. Judging from the changes of the shell length frequency distribution of the population, the members of the population except small individuals just after the recruitment had been subject to a strong mortality factor for some reason during the warm seasons since 1 April 2018.
Scarce recruitment to the population in the spring in 2019
The population remained in the extremely low density of less than 410 ind. m-2 between 27 December 2018 and 21 April 2019, since the numbers of the recruits that should be recognized as Cohort 7 and Cohort 8 were scarce after both of the breeding seasons in the spring and autumn.
Discussion
Factors Controlling the Population Dynamics of Asian Mussels
In this study, Asian mussels suffered from severe population decline in two different seasons (the summer in 2017, and the autumn in 2017 to the winter in 2018 and the autumn in 2018 to the winter in 2019). In the former case, the dense patches of Asian mussels with the density of 11,290 ind. m-2 and biomass of 5,186 gww m-2 on 28 May 2017 rapidly declined to less than one third of the density and about a quarter of the biomass by 21 August (Figure 5a, 6a). This species has a breeding season in July and mass recruitment of juveniles should occur to the population as it did in July to August 2018, but the number of the recruits was much restricted in 2017 (Figure 7a. In contrast, the population of short-neck clams fluctuated stably, even increasing the biomass three time larger in the same period (from 544 gww m-2 to 1,548 gww m-2). Although the habitats of these two species totally overlap on the tidal flats, the utilization in the micro-habitat level is markedly different between them. Asian mussels create muddy carpets on the sediment [38], while short-neck clams burrow the sediment [39]. Therefore, this event indicates that some strong mortality factor acted on only the benthic animals that occurred on the surface of the sediment, but it did not happen during the same season in 2018.
The seasonal fluctuations of the particle size composition of the sediment give a big hint to specify the cause of the rapid decline of Asian mussel population. The mud content of the sediment noted 41.3 % on 28 May 2017 due to the creation of muddy carpets by Asian mussels (Figure 4a), but it decreased to only 9.8 % on 25 July (Figure 3), when most of the muddy carpets disappeared and bare sandy surface appeared on the tidal flats (Figure 8a). This event indicates that a strong physical disturbance happened on the sediment, and most of the muddy carpets were washed away sometime in this period. According to the weather records at the local meteorological observatory in Kumamoto City, which is about 9 km apart from the study area, strong wind with 29.0 m·s-1 of the maximum instantaneous wind speed blew on 4 July 2017 due to a typhoon approached to the study area [40]. It is very likely that the muddy carpets with dense patches of Asian mussels were removed extensively from the tidal flats by the strong wind and waves caused by the typhoon.

In the latter case of the rapid decline of Asian mussel population during the autumn and winter, this event first reported on the present study area during the late autumn in 2014 and winter in 2015 [17]. The patches with the density of about 24,000 ind. m-2 and biomass of about 4,010 gww m-2 formed on 26 November in 2014 drastically decreased to the ones with 100 ind. m-2 and less than 10 gww m-2 by 8 March 2015, although no causes were not noted. At first, we suspected the possibility of the influence of severe winter weather. However, the weather in November and December in Kumamoto when this event starts is usually still warm, and the lowest daily mean temperature during the winter had never fallen below 0 °C except one day (-0.4 °C) in January 2011 in the past two decades [41]. It would be hard to attribute the rapid decline of the population of Asian mussels to the influence of intolerable low temperature.
In the follow-up study, we found that a large group of dabbling ducks including Anas platyrhynchos and An. acuta visited the tidal flats for feeding during low tides (Figure 8b), and the shells of Asian mussels and small juveniles of short-neck clams were found in the stomach contents of a dead individual of An. platyrhynchos) (Figure 8c). Every year, a large group of the ducks migrate from Siberia to Kumamoto for wintering [42]. It is very likely that the migratory ducks recently give a serious predation impact on the population persistence of Asian mussels and short-neck clams on the tidal flats during the late autumn and winter. In the previous studies on the feeding behaviors of the ducks on the Japanese coasts, Yamamuro et al. [32] found that three species of diving ducks (Aythya fuligula, Ay. ferina, and Ay. marila) fed mainly on bivalves including Asian mussels in estuarine lagoons that face Japan Sea, and gave a serious predation pressure on the bivalves during the winter.
On the tidal flats and near-shore areas in western Japan, nori cultivation fields are established extensively during the winter, which recently suffer from serious feeding damage by both of dabbling and diving ducks, and shells of the bivalves including Asian mussels were found with nori from their stomach contents [33-35]. Nori cultivation is popular on the tidal flats in the coast of Ariake Bay of which are the centers of the nori cultivation in Japan. All tidal flats of the bay including the present study area seem to be ones of the convenient feeding sites for the ducks during the low tide since they are able to feed on bivalves just walking on them (Figure 8b).
Factors Controlling Population Dynamics of Short-Neck Clams
The results of the population study of short-neck clams in this study indicates that it has suffered from a serious mortality factor during the spring and summer, and the first appearance of its negative impact on the population was the selective elimination of the individuals with the shell length of more than 12 mm from the population on 17 May 2018 (Figure 7b), which resulted in the rapid decline of biomass of the population (Figure 6b). During this period, we found many feeding traces marked by the rays on the sediment around the sampling site (Figure 8d). The same situations have been recently reported from various clam harvesting grounds on the tidal flats in western Japan, where the rays including A. flagellum and H. akajei gave a serious damage on the fishery activities [12,43]. Consequently, the dense patches of the clam with 34,170 ind. m-2 and 3,572 gww m-2 established on 1 April 2018 declined to ones with only 300 ind. m-2 and 61.1 gww m-2 by 17 October 2018 (Figure 5a,6a).
Such influence as depressed clam patches, however, did not occur during the warm seasons in 2017. The relatively high-density patches with 1,320 to 4,310 ind. m-2 and 452 to 1,548 gww m-2 were contrastively kept between 27 April and 8 November in 2017 (Figure 5a,6a). In the first half of this period, the clam patches were established in the muddy carpets created by Asian mussels (Figure 4a). It is likely that they provided a space refuge from the predation by the rays for the clam. In the latter half of this period, the muddy carpets were accidentally swept out from the tidal flats due to the strong wind and waves brought from the typhoon (Figure 4b). Therefore, the clam patches were not subject to a mortality factor caused by the development of reduced conditions in the muddy carpets as we had expected as a negative interference brought from Asian mussels to short-neck clams during the summer [15]. Thus, the accidental occurrence of the physical disturbance on the sediment caused by surf conditions may have worked as a key factor for the co-occurrence of Asian mussels and other macro-benthic animals including short-neck clams that favor oxidized sandy sediment.
Effective Measures to Re-establish Dense Patches of Short-Neck Clams
To re-establish dense patches of short-neck clams on the tidal flats is one of the most important issues for the sustainable development of the coastal fisheries in Japan [5]. Since this species originally predominates the macro-benthic communities on the sandy tidal flats across Japanese coast, the recovery of its population indicates to regain various functions for material circulation performed as a suspension-feeding bivalve in the ecosystem on the tidal flats [44]. However, it is apparent that the clam populations are placed under serious threats of mortality factors including predation by ducks, rays, snails etc. and there being enforced interspecific interaction with Asian mussels that have happened in the recent years.
Nevertheless, the members of the fisherman’s Associations have demonstrated by the net bag experiments that the capacity of the secondary clam production is potentially still kept intact on the tidal flats where the dense patches of the clam had disappeared. They put net bags with small pieces of oyster shell or gravels on the tidal flats to induce the settlement of planktonic larvae of the clam on the substrates inside the bags (Figure 9a). In fact, the planktonic larvae settle on the substrates, and can grow normally without any predation as they receive outside the bags (Figure 9b). Although we need to further modify this method to develop as cost-effective way, it gives as a big hint how to re-establish dense patches of short-neck clams on the tidal flats as the 1970s.

Conclusion
Two species of bivalves, Asian mussels (A. senhousia) and short-neck clams (R. philippinarum) predominated the macro-benthic community on Midori River Tidal Flats. In the former species, innumerous planktonic larvae settled on the sediment in the breeding season of early summer, and dense patches were established, creating muddy carpets on the sediment. However, they rapidly declined due to the physical disturbance on the sediment caused by strong wind and waves when a typhoon passed through near the study area, and due to predation by the migratory dabbling ducks during the late autumn and winter. Although the creation of muddy carpets by Asian mussels expected to develop reduced conditions in the muddy carpets during the summer, and to work as a serious mortality factor to short-neck clams, the physical disturbance of the sediment caused by a typhoon swept out the muddy carpets themselves from the tidal flats. The latter species burrowed the sediment and showed a high resistance to the disturbance. However, it seriously suffered from the predation by rays during the spring and summer. Consequently, very poor macro-benthic communities were formed in both of density and biomass on the tidal flats. To recover the clam-harvesting fishery on the tidal flats, short-neck clams need to be protected not only from the development of reduced conditions in the muddy carpets during the summer as expected prior to this study but also predation by the rays.
To Know More About Oceanography & Fisheries Open Access Journal Please click on:
https://juniperpublishers.com/ofoaj/index.php
For more Open Access Journals in Juniper Publishers please click on:
https://juniperpublishers.com/index.php

Comments
Post a Comment