Zooplankton Diversity and Composition of Malampaya Inner Sound, Taytay, Palawan, Philippines
Oceanography & Fisheries Open Access Journal Juniper Publishers
Abstract
Zooplankton samples were taken in six sampling stations at Malampaya Inner Sound, Taytay, Palawan, to determine its abundance, distribution, and diversity, and composition related to the complete lunar cycle during the month of April 2007. Horizontal towing and vertical hauling of 333 μm plankton net was conducted from the afternoon to midnight, two hours before the onset of the moon phase. 27 zooplankton taxa belonged to eight (8) different phyla identified. During the entire lunar phase, arthropods, chordates, cnidarians, and chaetognaths were present. Zooplankton appears in great abundance during nighttime, full moon, and high tide based on 1-month sampling. Higher zooplankton diversity was observed during the new and full moon, largely represented by arthropods, particularly copepods and decapods. Generally, the area has a high number of individuals but low in diversity. A more extended sampling period and more sampling events should be conducted in the area to correlate zooplankton abundance and composition among lunar phases. Wind and current pattern and nutrient composition should be taken to better understand the plankton productivity in Malampaya Inner Sound.
Keywords: Zooplankton; Abundance; Diversity; Lunar cycle
Introduction
Plankton are organisms that live suspended in the water column and drift with the current. They include protists, animals, plants, and bacteria, where most are microscopic. Zooplankton (animal plankton) are also known as the primary consumers in the oceans that primarily feed on phytoplankton (plant plankton) and serve as food for higher trophic level organisms. Although they are small, their great abundance and diversity can support larger animals. Estuarine is important, ecologically, and physically, i.e., their value to human welfare. They are used as a waste disposal site that continually enriches them with nutrients, creating conditions of exceptionally high productivity. It serves as the region essential to many marine organisms’ life history and development, particularly on their larval stages (meroplankton) and host commercial and recreationally important finfish and shellfish. It is also a vital breeding ground or nursery region [1]. The presence of high productivity may indicate an abundance of higher trophic level organisms provided by an essential index of biological production in estuaries [2]. It was reported that representative genera of estuarine zooplankton include the copepods, mysids, and amphipods [1]. Zooplankton in estuaries is generally represented by organisms belonging to phylum Arthropoda, mainly composed of copepods. Copepods are the net zooplankton in the oceanic system [2].
As an estuary, such as Malampaya Sound may harbor a large population of zooplankton, which could initially explain the area’s high fish population, i.e., the “fishbowl of the Philippines” during the 1950s [3]. Various studies and reports in Malampaya Inner Sound indicate the abundance of commercially important aquatic resources in the area. The presence and abundance of plankton play a significant role in supporting the large fish population in the area. However, studies on these minute species that serve as food for bigger marine organisms are limited. This paper presents the Malampaya Inner Sound zooplankton diversity, composition, and abundance based on lunar phases.
Materials and Methods
Sampling area and station
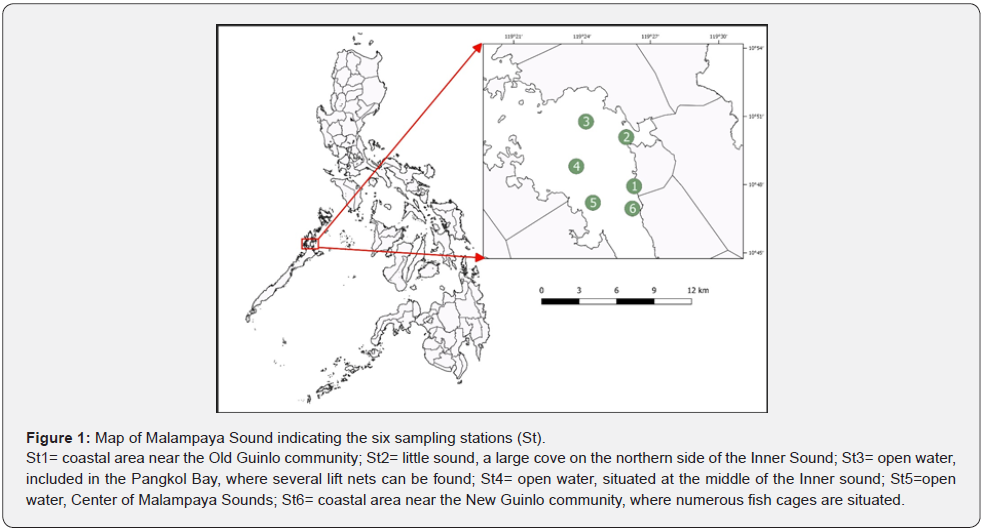
The study was conducted in Malampaya Inner Sound, located in the western part of the province, adjacent to El Nido, Palawan, Philippines, covering about 200,155 ha. It is 14.4km long and 6.4km wide, with depths of 5.5 to 16.5m in the middle. The sound offers significant ecological features and livelihood opportunities for its surrounding communities. Most of the population is primarily engaged in fishing and secondarily in farming. Others are involved in agriculture and seaweed culture and the culture of fish in cages. Interestingly, it is the only known habitat for Irrawaddy dolphins in the Philippines. Six sampling stations (Figure 1) were initially established using the Geographical Positioning System (GPS) during a preliminary survey. The characteristic of each site served in the identification of sampling stations. Two sampling stations were in highly populated areas or near the communities (Stations 1 and 6) and another from a cove (Station 2), protected from the strong current. The remaining three sites were established in the open water (Stations 3, 4, and 5).
Sampling method and analysis
There were four sampling events within a month. Horizontal towing and vertical hauling of 333μm plankton net was done approximately two hours before the onset of the moon phase. Horizontal towing parallel to the coastline was done for 10 minutes in every sampling station at a speed of approximately 1.0 m/s. After towing, the plankton net was retrieved, then washed down from the outside, or the net was lowered to the water several times to allow plankton to be washed down to the cod-end bucket below, and the sample was kept in a bottle with a label. Vertical hauling was also conducted. The plankton net was lowered 2-3 minutes in the water and then retrieved as was done with the horizontal tow. The 5% buffered formalin solution was used to preserve the samples prepared and stored for a week before the scheduled sampling. Labels were placed into the bottles, where samples were kept. All samples collected were brought to the WPU-PPC laboratory. Identification was done using the works of [4-7]. Relative compositions, abundance, and distribution were determined for every station and compared among the different lunar phases. Both abundance and distribution were analyzed and expressed in individual/ml. Species diversity was derived using the Shannon-Weaver index [8].
S
H’= -Σ P log2 Pi
i =1
Where: S= is the total number of species
Pi= is the proportion of the number of species i to the total of individual, that is P i= n i / N; then one might as well cast the equation for H’ directly in terms of the observed values of ni.
N = is the total number of individuals; n1, n2…n3 are the respective value of individuals of each species
Results and Discussion
Zooplankton taxa
A minimum of 27 zooplankton taxa belonged to 20 families and eight phyla, namely: phylum Arthropoda, Chordata, Cnidaria, Chaetognatha, Echinodermata, Mollusca, Granureticulosa (Foraminifera), and Annelida. A total of 49 genera were identified, while seven were unidentified. Organisms under the phylum Arthropoda have the largest number of zooplankton representatives in the entire sampling event. The zooplankton taxa in the area are consistent with the findings of [3], although they collected their samples during the daytime. In their study, the zooplankton of Malampaya Sound belongs to seven different zooplankton phyla, namely, Arthropoda, Chordata, Annelida, Chaetognatha, Cnidaria, Mollusca, and Echinodermata. In the present study, phylum Granureticulosa (Foraminifera) was collected and the above-mentioned phyla. The high number of representatives belonging to the phylum Arthropoda supports the fact that Arthropoda is the largest phylum in the animal kingdom [9]. Among macrobenthos, crustaceans, polychaetes, and mollusks commonly dominate the estuarine plankton communities [1]. It is common to find copepod species such as Acartia, Centropages, and Eurytemora. The largest number of different copepods taxa/genus found in the area is expected since these organisms are the net zooplankton in the oceanic ecosystem particularly in an estuarine environment as cited by [9]. According to [3], copepods are the primary zooplankton found in the area. The cladocerans (Evadne and Penelia), ostracods (Cypridina), and decapods (Lucifer) mostly in larval stages, mysis, zoea, shrimp larvae, brachyuran zoea, and megalopa larvae form the other components of phylum Arthropoda in the area. Many larval forms in which organisms, particularly arthropods, pass through different larval stages before becoming an adult probably explain the dominance in several this group partly [1]. He also added that light, which occurs 12 hours a day in the tropics, is the principal factor that influences molting, spawning, and growth of estuarine fauna. Another reason for these high larval forms is that coastal nekton commonly uses estuaries as a nursery ground, probably for the advantage of protection and abundance of food as cited by [3]. Collected organisms such as the chaetognaths or the arrow worms, annelids, echinoderms, mollusk, and cnidarians, coincide with those organisms collected by in their study. It indicates that they are a permanent zooplankton resident in the area.
Abundance and Distribution
With horizontal towing, zooplankton population was relatively higher in stations 1, 5 and 6 (>30 ind/ml) compared with stations 2, 3 and 4 (<25 ind/ml). The greatest abundance of 68 ind/ml zooplankton was observed at station six, as indicated in Figure 2, which occurred largely during full moon and last quarter samplings. It was followed by station 1 (Guinlo), which had 35 ind/ ml. With vertical hauling, station 4 has the highest zooplankton abundance (17 ind/ml), followed by 16 ind/ml at station 6. It was observed that horizontal and vertical abundance except in Station 6 were somewhat inversely proportional, i.e., if abundance in the station of horizontal towing was high, its vertical samples have lower abundance. Another observation is the sudden zooplankton increase during the last quarter, particularly at stations 5 and 6 when tide level ranges are at their maximum.
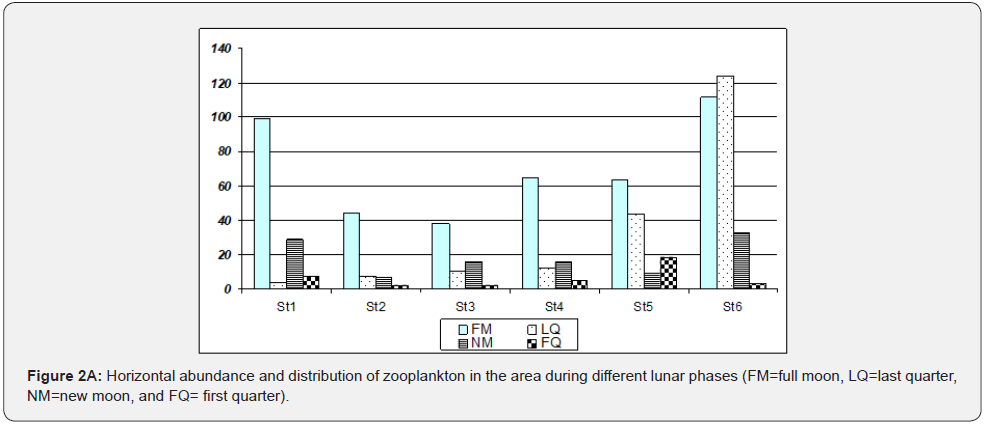
Zooplankton also tends to be collected more abundantly using horizontal towing. In terms of sampling events, great abundance occurred during the first sampling event (full moon) and decreased as the sampling event progressed (Figure 3). Plankton abundance varies horizontally, vertically, and seasonally. As indicated in the results, Station 6 has the highest abundance. This large population of zooplankton in the area, especially during the full moon, last quarter (horizontal towing), and new moon (vertical hauling), may be due to sampling time, tide levels, and other characteristics. The towing distance is more important than the size of the net in determining the accuracy of the samples as cited by [10]. Sampling events correspond with nighttime (1700 to 0200 HR) and varying tide levels. Higher abundance was noted using horizontal towing since, during nighttime, zooplankton tends to migrate at the surface, preferably to feed and avoid predators [2]. Zooplankton migrates upward during the night and descends during the day [11]. Tide levels have a relationship with zooplankton abundance; as tide level increases, zooplankton abundance increases, and low tide level may indicate less zooplankton population [12]. It was reported by that as the tide level increases, organisms migrate into the shallower areas of tidal flats, searching for food. As the tide recedes and pressure declines, these organisms retreat toward deeper water.
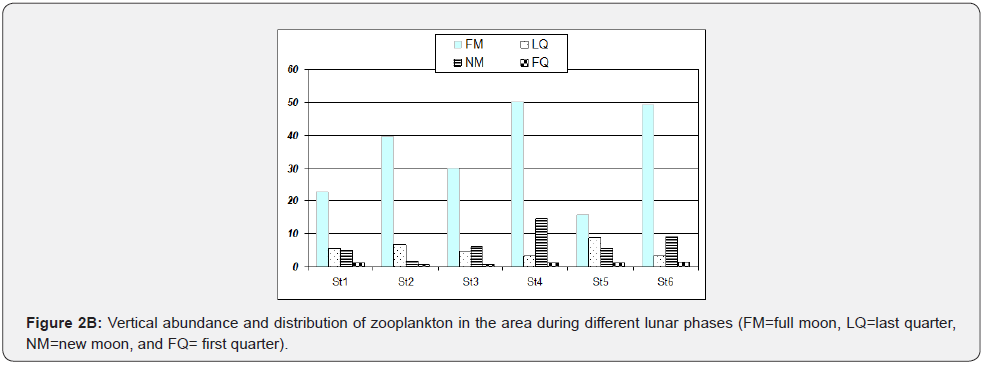
The highest abundance at station six can be attributed to the stations, location, and sampling site characteristics. The area is situated near shore and near human communities, which has something to do with phytoplankton production. Zooplankton migrate to the surface layer where phytoplankton are rich to feed on rich phytoplankton production. Phytoplankton need dissolved nutrients, sunlight, and other factors which could be found on the surface to be used for photosynthesis [2]. Another reason is that the area is in coastal waters; as cited by [13], phytoplankton production is enhanced at any latitude in coastal waters. He also emphasizes that landmasses of tropical effects can directly influence the abundance and higher plankton production in coastal waters of tropical regions, which can be attributed to the input of nutrients, trace metals, and organic matter. Humic substances and fecal wastes from near human communities and fish cages may be partly associated with this high abundance of zooplankton. Increased nutrient availability due to run-off and regeneration in shallow-water, stabilization, and other factors are involved [14]. The area receives large amounts of nutrients and organic nutrients from the land through river run-off, absorbed by phytoplankton and consequently grazed by zooplankton. Station 1 followed station 6 in terms of abundance with 43 ind/ml. Most of the samples were collected during the full moon and new moon (horizontal), and last quarter (vertical). Unlike station 6, station 1, although characterized as a community-near area, is not heavily populated, and no river can be found in the area. There are also fewer fish cages compared with Station 6. Stations 4 and 5 are in the open water and have an abundance of 42 ind/ml (horizontal + vertical). This abundance may be due to the area’s characteristics, which is deeper (10m); hence, many individuals were collected. A larger area was covered for sampling. Zooplankton distribution is affected by depth, proximity to landmass, nutrient levels, biotic interactions, and indirectly by diurnal migrations as cited by [14]. Stations 2 and 3 have 27 ind/ml (horizontal + vertical), although most samples were from horizontal towing.
The increase in zooplankton at stations 5 and 6 during the last quarter sampling can be attributed to the mild water current observed during the sampling. The distribution of zooplankton being suspended in water is affected mainly by circulation patterns and wind directions as cited by [14]. According to [3], Malampaya Sound has two pronounced seasons; dry from November to April (northeast monsoon) and wet during the year. Since stations 6 and 5 are found the opposite to the sounds’ opening to the open sea and on the southeast part, there could be a possibility that zooplankton may be driven by a strong wind that induced current during sampling event, causing a sudden increase of zooplankton in these stations. In line with this, [14] in their study of the Ragay Gulf, Philippines, reported that zooplankton tends to increase during the northeast monsoon. However, this study cannot be confirmed since there is no available data for current and wind patterns during the sampling period.
The observed decrease in zooplankton abundance as the sampling event proceeded from full moon to first quarter may be due to the sampling period (April) and phytoplankton production. The spring (February/March) increase in light and water temperature initiates a spring phytoplankton bloom in mid-latitude estuaries [1]. In relation to this, [15] states that zooplankton appears in great abundance after phytoplankton bloom has passed. Zooplankton rise in middle March to April throughout the sea as cited by [16]. In Florida, maximum production rates usually occur during the summer months due to the strong development of holoplankton and meroplankton [1]. The observed dominance of arthropods in zooplankton samples except during the first quarter in which chordates exceed arthropods might be because arthropod is the largest animal phylum where many zooplankton organisms belong [9]. It also has the highest representative taxa in the entire sampling event. The copepods and decapods constantly dominate many Arthropods in the area [3]. The higher number of individuals during full moon sampling is due to the sampling event conditions and samples collected.
Both tide level and sampling during night trigger zooplankton abundance attributed to the tendency of zooplankton to migrate and increase during high tide levels. According to [12], when the tide was highest, zooplankton increases. Chordates tend to increase during the last quarter and exceed the number of arthropods during the first quarter due to the bulk samples of ichthyoplankton (eggs and larva) and Oikopleura sp. This event can be related to the sampling season temperature. Summer (May) is associated with the increased temperature that strongly influenced the development of fishes which are poikilotherms. The maximum production usually occurs during the summer months due to the strong development of holoplankton and meroplankton [1]. He added that light, which appears 12 hours a day, is the principal factor influencing the molting, spawning, and growth of estuarine fauna. The presence of mollusks and annelids during full moon and last quarter and forams during full moon indicates that these organisms require certain tide levels to migrate. Cnidarians increased as the sampling event proceeded, particularly jellyfish larvae. This could be attributed to the season and temperature. According to Tandoc (pers. comm.) jellyfish tend to increase during summer (April-May). As evidence for this increasing jellyfish population, during this study, a jellyfish factory in the area resumes its operation simply because jellyfish are abundant during this time. Chaetognaths consistently occur in all sampling events. This can be explained by the abundance of their food source since chaetognaths’ primary foods are copepods [1].
Zooplankton composition
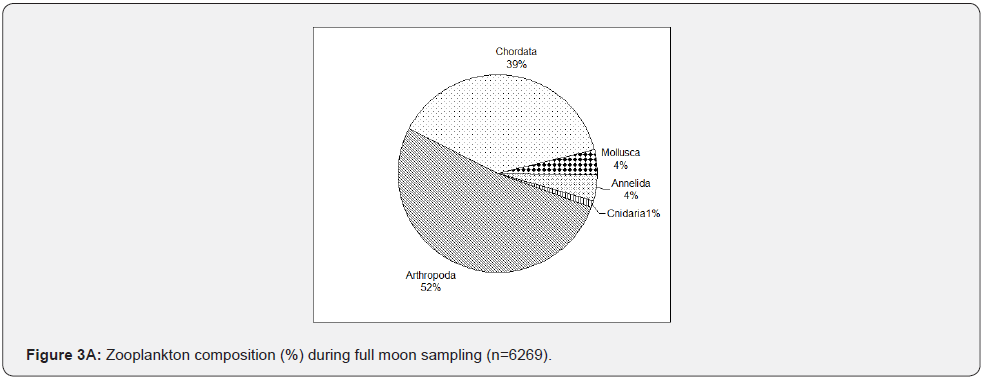
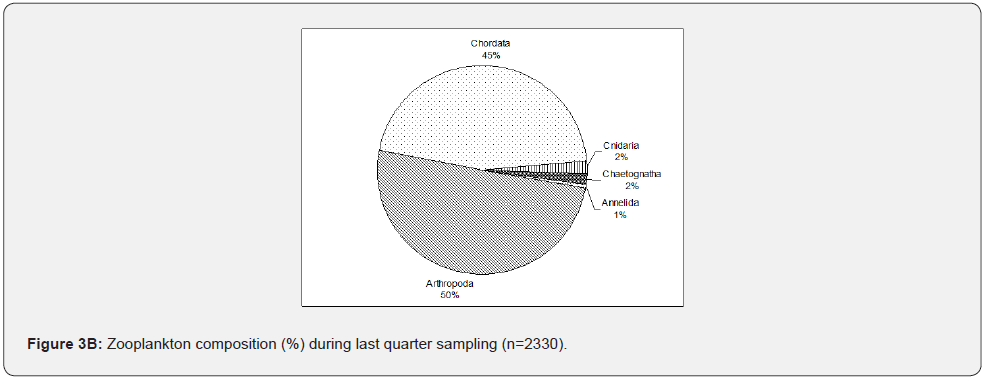
As shown in Figure 3A, during the full moon, zooplankton was represented by eight different phyla. A large proportion of full moon samples consist of phylum Arthropoda (>50%), particularly copepods (Oithona, Centropages and Acartia, cladocerans (Penelia.), decapods (Lucifer & Brachyuran zoea), and barnacles (Balanus). This was followed by phylum Chordata (39%), consisting of fish eggs, larvae, and Oikopleura sp. Phylum Mollusca and phylum Annelida occurred with a minimum number of individuals collected (4%). Phylum Cnidaria (1%) includes jellyfish larvae and Foraminifera, Chaetognatha (Sagitta), and Echinodermata comprised the remaining parts of the samples (<1%). With fewer samples collected than full moon sampling, zooplankton composition during the last quarter (Figure 3B) did not greatly vary with the full moon results, i.e., samples were constantly dominated or a large fraction of samples belong to phylum Arthropoda except for the increase of phylum Chordata (45%), which is mainly due to the Oikopleura spp. and ichthyoplankton. Phylum Cnidaria, especially jellyfish larvae, tend to increase and are as abundant as Chaetognatha (Sagitta) (2%). In contrast, phylum Annelida decreased as phylum Mollusca reduced in numbers. Foraminifera were not collected again during the last three sampling events. Phylum Echinodermata was also not present during the last quarter sampling.
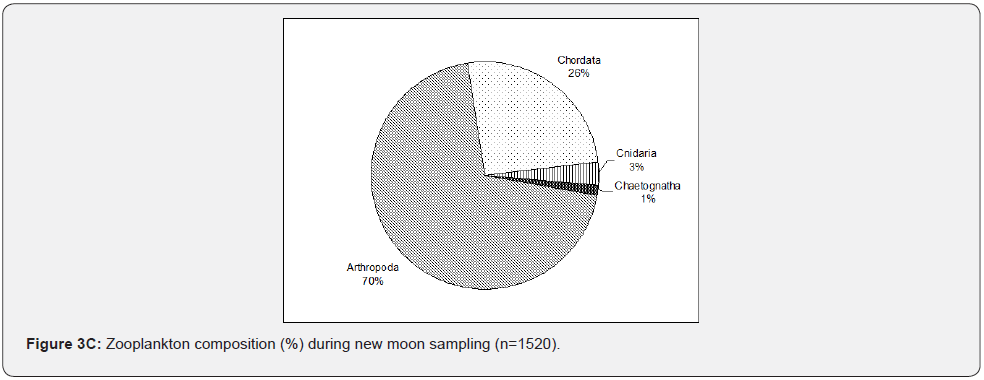
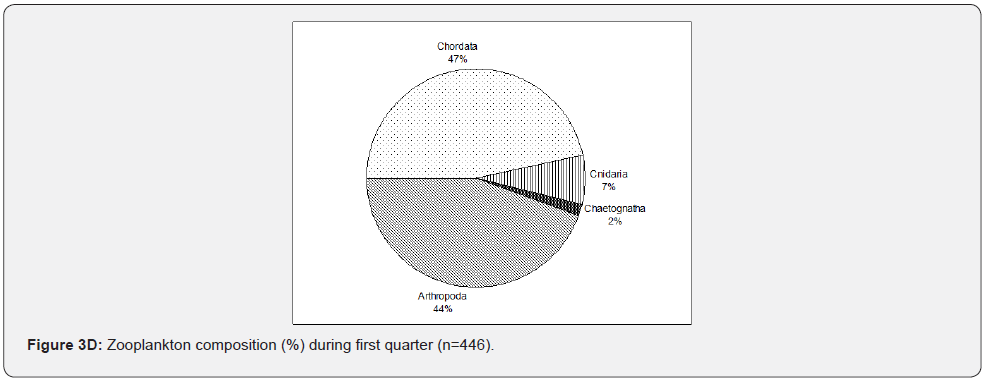
In Figure 3C, during new moon sampling, which is the same as other sampling events, large samples collected belong to phylum Arthropoda with an increasing number (70%) than the other sampling events. Phylum Chordata (26%) consistently followed this large dominance of phylum Arthropoda. Chaetognatha consistently occurred with lesser (1%) individuals collected. Phylum Echinodermata reappeared during new moon sampling. This was an interesting incident but needed further investigation for its explanation. Phylum Cnidaria notably increased during this sampling event (3%). In the first quarter (Figure 3D), unlike other sampling events, phylum Chordata dominated the collected samples (46%) while phylum Arthropods (44%) was only second in abundance. Chaetognatha continues to occur, slightly increased to 2%, and Cnidaria further increased to 7%. Phylum Echinodermata has less than 1%, Annelida, Mollucsca, and Foraminifera were not collected during this sampling event. Zooplankton tends to decrease as sampling events proceeded, starting from the full moon towards the first quarter.
Zooplankton composition varied from one event to another based on different lunar phases. The Arthropoda tends to increase during the full moon and new moon samplings. In contrast, the Chordata tends to increase during the last quarter and dominates during the first quarter over Arthropoda. Comparing all the sampling events, the Cnidaria increases from the full moon to the first quarter. The Echinodermata was absent last quarter, and Chaetognatha was consistently collected in the sampling events. Still, Foraminifera, Mollusca, and Annelida were only taken during the full moon and last quarter (Figure 4), which coincide with the high tide. The analysis is made only on taxonomic or ecological groups of organisms thought to be important and easy to identify. It is impossible to measure the diversity of an entire community considering all species [8]. The observed high diversity during new moon and full moon sampling may correspond to the sampling time, conditions, and samples collected, i.e., nighttime sampling, its tide levels, and the samples’ composition
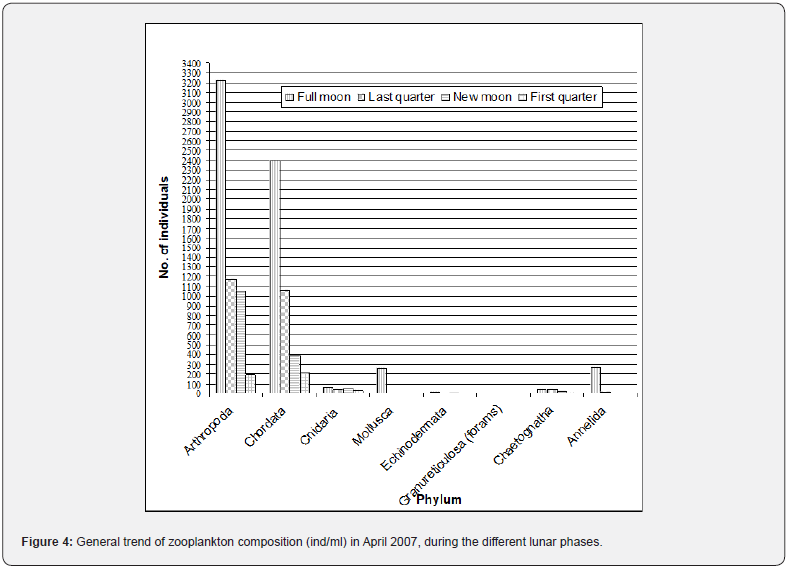
Although fewer individuals are present during the new moon than the full moon and last quarter, it has the highest diversity noted on both sampling methods. Most samples belong to phylum Arthropoda during new moon sampling, composed of more than 20 zooplankton taxa, such as the different decapods and copepods. Unlike full moon and last quarter, which has a high abundance or number of collected zooplankton, samples were high. Still, one or two zooplankton taxa just dominated these. The population with a high number of species (taxa) but occur without clear dominance has higher diversity than the low diversity population there are few dominating species or taxa [13]. This statement is true or the same with the results of this study in which full moon and new moon are abundant but dominated by only a few taxa while last and first quarter has less but without domination on its composition. The full moon, which has the 39 zooplankton taxa identified with the highest abundance, ranked only second in diversity. This is due to some taxa that have a meager individual (<5 ind. during the entire sampling period) and those taxa such as Oikupleura, eggs, and copepods that dominate the samples. Thus, there is an imbalance in the population, causing the samples to have lower diversity than the new moon. The diversity decreases when a community becomes dominated by one or fewer species [8]. Diversity tends to increase with an increase in the number of species (taxa), and hence, the number of samples is true in the case of the full moon.
In parallel with a full moon and new moon sampling, the first quarter, which has fewer taxa and samples collected, becomes higher in diversity than last quarter, which is abundance next to the full moon number of samples. These results also correspond to the first reason, i.e., the dominance of species or taxa in a population or samples can cause low diversity. During the last quarter, samples collected are dominated mainly by eggs, barnacles nauplii, Oikopleura sp, and copepods have less diversity compared to the first quarter. Although with fewer species and individuals, the first quarter had a proportionate distribution of samples for every taxon, indicating higher diversity. The higher diversity during the new moon and full moon than the first and last quarter is due to samples collected. Most of the samples belong to the phylum Arthropoda, which comprises many representative taxa during the full moon and new moon. Chordates, composed only of ichthyoplankton with only five representatives, increased and dominated the samples during the last and first quarters. Another possible cause for this high diversity of full moon and the new moon is the incidence of spring tides during these lunar phases.
Horizontal and vertical diversity
Diversity for horizontal towing was higher during new moon and full moon sampling events than last and first quarters. This high diversity was observed from the new moon first, followed by the full moon, first and last quarters. Zooplankton abundance was higher during the full moon and last quarter while less abundant during the new moon and first quarter, opposite to that in diversity index (Table 1). The same with diversity in horizontal towing, the new moon consistently had the highest diversity, followed by the full moon, first quarter, and last quarter. It is also in contrast with the abundance results, in which full moon and last quarter had the highest abundance while new moon and first quarter were lesser.
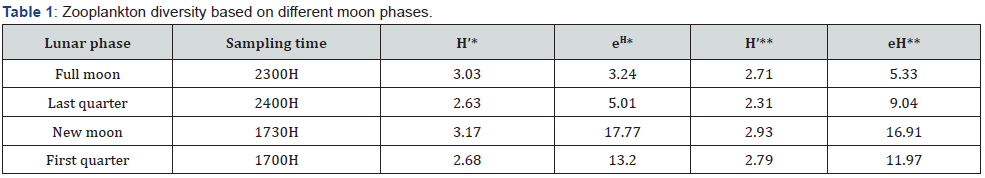
H’- species diversity; eH- species evenness; * - horizontal sampling; **- vertical sampling
In parallel with full moon and new moon sampling, first quarter which has a smaller number of taxa and samples collected becomes higher in terms of diversity than last quarter which is in terms of abundance is next to full moon number of samples. These results also correspond to the first reason, i.e., dominance of certain species or taxa in a population or samples can cause low diversity. During last quarter, samples collected are dominated mainly by eggs, barnacles nauplii, Oikopleura sp, and copepods have less diversity compared to first quarter. First quarter, although with a smaller number of species and individuals, had a proportionate distribution of samples for every taxon indicating higher diversity. The higher diversity during new moon and full moon compared to first and last quarter is due to samples collected. During full moon and new moon most of the samples belong to phylum Arthropoda which are composed of many representative taxa. Chordates, which is composed only of ichthyoplankton with only 5 representatives increased and dominated the samples during last and first quarters. Another possible caused for this high diversity of full moon and new moon is the incidence of spring tides which occur during these lunar phases, when tide level ranges are at its maximum.
Conclusion and Recommendations
Based on the results, most of the collected samples during the present study coincided with the organisms, which were also found by [3]. Spring phytoplankton bloom triggers zooplankton abundance. Human communities and current patterns affect zooplankton abundance. Arthropods (53%), mainly copepods, decapods, and chordates (38%) such as ichthyoplankton and Oikopleura , are the major zooplankton found in the area. Zooplankton decreases as the sampling event proceeded (full moon, last quarter, new moon, and first quarter). Arthropods are the most dominant during the full moon and new moon, while chordates during the first and last quarters. Sampling time, conditions influence the diversity of zooplankton in this study and samples collected. A more comprehensive correlation study must be conducted, at least three months, to determine whether there is a relationship between zooplankton composition and its abundance regarding the various lunar phases. Physical and chemical parameters, particularly nutrient rates composition such as nitrogen, phosphorus, silicon, and wind direction or current pattern, should be carried out to understand better the plankton’s productivity, which characterizes the area’s overall productivity.
To Know More About Oceanography & Fisheries Open Access Journal Please click on:
For more Open Access Journals in Juniper Publishers please click on:

Comments
Post a Comment