Effect of Origanum vulgare Extract on Immune Responses and Heamatological Parameters of Rainbow Trout (Oncorhynchus mykiss)- Juniper Publishers
Juniper Publishers- Journal of Oceanography
Abstract
In this study, Origanum vulgare extract was used to evaluate its effects on immune responses and hematological parameters of rainbow trout (Oncorhynchus mykiss). Six hundred (600) averages mean weight 13±0.05g rainbow trout (Oncorhynchus mykiss) were randomly allocated into two groups including placebo-treated group (control), and Origanum vulgare extract-treated group, each of three replicates. The fishes were hand-fed once a day with diet medicated placebo or Origanum vulgare extract (OE) at a rate of 1% of feed weight in the first feeding for 8 weeks. At the end of every two weeks 24hrs after feeding, fish were bled from caudal vein and blood samples were analyzed for some of hematological and immunological parameters. The results showed that serum total protein, albumin and globulin, respiratory burst activity, phagocytic activity and serum lysozyme activity vary among the two treatment groups which were found to be higher in OE-treated group (P<0.05). It was concluded that supplementation of OE at a rate of 1% registered higher immunological responses. Therefore, dietary inclusion of OE could improve nonspecific immune responses in rainbow trout. Future studies to determine optimal herb mixtures and dietary levels should be conducted.
Keywords: Herbal immunostimulant; Iranian medicinal plants; Origanum vulgare; Fish
Introduction
Major targets in the aquaculture industry are to maintain fish health as well as to improve fish performance. The use of plant extracts in practical diets for fish is a very topical concept in aquaculture industry. One of the most important aspects in rainbow trout farming is the nutrition factor. It can influence the performance as well as the health status of the cultured fish. Origanum vulgare is a member of the Labiatae family of plants. It is an aromatic plant with a wide distribution throughout the Mediterranean area and Asia [1]. The essential oil obtained from O. vulgare subsp. hirtum plant by a steam distillation process comprises more than 20 ingredients, most of which are phenolic antioxidants [2]. Major components are carvacrol and thymol that constitute about 78 to 82% of the total oil [3]. It has been suggested that the essential oil derived from oregano possess in vitro antimicrobial [4,5] antifungal [6], insecticidal [7] and antioxidant [8] properties. These properties are mainly attributed to carvacrol and thymol. The activity of other constituents such as the two monoterpene hydrocarbons, γ-terpinene and p-cymene, that often constitute about 5 and 7% of the total oil, respectively [3] is uncertain.
Materials and Methods
Preparation of Origanum vulgare extract
The plant of Origanum vulgare was procured from local store and plant species was identified and confirmed by a botanist in Institute of Medicinal Plants. The dried air parts were collected and washed in sterile distilled water. The samples were separately shade-dried for 10 day till weight constancy was achieved. Then, the samples were powdered in an electric blender. The extract was prepared with the standard method of percolation. To do this, chopped dried air parts of plant in 80% ethanol were percolated for 72 hours. Then, the slurry was filtered with Whattman No. 1 filter paper and centrifuged for 5min at 5000rpm. The filtrate obtained from ethanol using a rotary device, the excess solvent was separated from the extract. These crude extract was stored at 4 °C until use.
Supplementation ofthe normal diet with dried Origanum vulgare extract
The formulated fish feed was prepared using the normal fish diet (50% crude protein, 18% crude lipid, 1.9% fiber, 1.3% total phosphorus, 8.3% ashes, and 14.8% nitrogen free extract) with dried Origanum vulgare extract or placebo at a ratio 1% of weight food and mixing part by part in a drum mixer. Sufficient water along with the oil ingredients were then added to make a paste of each diet. After it was pelleted and allowed to cool dry. The pellets were air dried and stored in air tight containers until fed.
Fish and experimental conditions
600 rainbow trout weighing 13±0.05 were used. All experiments were carried out in 1,000 liter round concrete ponds with a continuous water flow of 2.5 liter per second. The fish were kept at an ambient, including uncontrolled water temperature of 15±1 °C, dissolved oxygen of 7.2±0.2mg l-1 and pH 8±0.3. After 2 weeks adaptation, fish were randomly allotted in two groups including an experimental group and a control group, in triplicate was maintained in 6 concrete ponds each containing 100 fish. Each group was hand-fed once a day with diet medicated 1% of Origanum vulgare extract, or placebo (70% lactose, 10 % starch and 20 % talc) prepared in the laboratory and three times with normal diet at a rate 2% of body weight for 10 weeks.
Bleeding and serum collection
During bleeding, fish were rapidly netted, tranquillized with 50mg/l of tricaine methane sulfonate (MS222, Sigma chemical Co. St. Louis, MO, USA). Fish were bled from caudal vein using 1ml insulin syringe fitted with 24 gauge needle. To minimize the stress to fish, 1ml of blood was drawn and the whole bleeding procedure was completed within 1min. A total number of 15 blood samples were collected from 15 fish in each group (5 samples from each replicate) at the end of every 2 weeks, 24h after final feeding period. The blood pooling of 5 fish from each replicate divided into 2 haves. Half collected in serological tubes containing a pinch of lithium heparin powder, shaken gently and kept at 4 °C to test hematological parameters. Other half collected in tubes without of anticoagulant and allowed to clot at 4 °C for 2hrs to test serological parameters. The clot was the spun down at 2000rpm for 10min to separate the serum. The serum collected by micropipette and was stored in sterile Eppendorf tubes at -20 °C until used for assay.
Hematological assay
Blood sample was analyzed with routine methods adopted in fish hematology [9]. The total red blood cell counts (RBC x106/μl) were determined in a 1:200 dilution of the blood sample in Hayem's solution and total white blood cell counts (WBC x103/μl) in a 1:20 dilution of the blood sample with a Neubauer hemocytometer. The hematocrit (Hct) and leucocrit percentages were determined in duplicate by using micro hematocrit-heparinized capillary tubes of 75μl volume and a micro hematocrit centrifuge at 15000rpm for 5min [10]. The percentages of erythrocyte (hematocrit) and leucocyte (leucocrit) volumes were calculated by overlaying the tubes on a sliding scale hematocrit reader.
The hemoglobin (Hbg/dl) concentrations were determined by the cyanomethaemoglobin method [11] using a haemoglobin reagent set (Ziest Chem Diagnostics). The all the values of red blood cell indices, the mean values of cell haemoglobin (MCH pg), cell hemoglobin concentration (MCHC %), and cell hemoglobin volume (MCV fl) were calculated according to Wintrobe formulae [12]. The differential leukocytes count was carried out using blood smears stained with Wright-Giemsa. The percentage composition of leukocytes was determined based on their identification characters listed by Ivanava [13].
Biochemical assay
Serum total protein content was estimated photo metrically by citrate buffer and bromocresol green (BCG) dye binding method [14] using the kit (total protein and albumin kit, Pars Azmun Company, Iran). Albumin was determined BCG binding method. The absorbance of standard and test were measured against blank in a spectrophotometer at 546nm. Globulin level was calculated by subtracting albumin values from total serum protein. Albumin/globulin (A/G) ratio was calculated by diving albumin values by globulin values.
Immunological assay
Separation of leukocytes from the blood
Leucocytes for assay were separated from each blood sample by density-gradient centrifugation. One milliliter of histopaque 1.119 (Sigma) containing 100μl of bactohemagglutination buffer, pH 7.3 (Difco, USA) was dispensed into siliconised tubes. 1ml of a mixture of 1.077 density histopaque and hemagglutination buffer and 1ml of blood was carefully layered on the top. The sample preparations were centrifuged at 2500rpm for 15min at 4 °C. After centrifugation, plasma was collected and stored at -80 °C for future analysis; separated leukocytes were gently removed and dispensed into siliconised tubes, containing phenol red free Hanks Balanced Salt Solution (HBSS, Sigma). Cells were then washed twice in HBSS and adjusted to 2*106 viable cells/ml.
Respiratory burst activity
Respiratory burst activity of isolated leukocytes was quantified by reduction of ferricytochrome C [15]. Briefly, 100μl of leukocyte suspension and an equal volume of cytochrome C (2mg/l in phenol red free HBSS) containing phorbol 12-myristate 13-acetate (PMA, Sigma) at 1μg/ml were placed in triplicate in micro titer plates. In order to test specificity, another 100μl of leukocyte suspensions and solutions of cytochrome c containing PMA and superoxide dismutase (SOD, Sigma) at 300U/ml were prepared in triplicate in micro titer plates. Samples were then mixed and incubated at room temperature for 15min. Extinctions were measured at 550nm against a cytochrome C blank in a multiscan spectrophotometer. Readings were converted to nmoles O2 by subtracting the O.D. of the PMA/SOD treated supernatant from that treated with PMA given alone for each sample, and converting O.D. to n moles O2 by multiplying by 15.87. Final results were expressed as nano moles O2 produced per 105 blood leukocytes.
Phagocytosis assay
Phagocytosis activity of blood leukocytes was determined spectrophotometrically according to Seeley et al. [16]. This assay involves the measurement of congo red-stained yeast cells which have been phagocytised by cells. To perform the assay, 250μl of the leukocyte solution was mixed with 500μl of the congo red- stained and autoclaved yeast cell suspension (providing a yeast cell: leukocyte ratio of 40:1). The mixtures were incubated at room temperature for 60min. Following incubation, 1ml ice-cold HBSS was added and1 ml of histopaque (1.077) was injected into the bottom of each sample tube. The samples were centrifuged at 2500rpm for 5min to separate leukocytes from free yeast cells. Leukocytes were harvested and washed two times in HBSS. The cells then were resuspended in 1ml trypsin-EDTA solution (5.0g/l trypsin and 2.0g/l EDTA, Sigma) and incubated at 37 °C overnight. The absorbance of the samples was measured at 510nm using trypsin-EDTA as a blank.
Serum lysozyme assay
In this study, an assay based on the lysis of Micrococcus lysodeikticus was used to determining the lysozyme activity. Serum lysozyme activity was measured spectrophotometrically according to the method Parry et al. [17]. Briefly, 0.02% (w/v) lyophilized Micrococcus lysodeikticus in 0.05mM solution phosphate buffer (pH 6.2) was used as substrate. 10μl of fish serum was added to 250μl of bacterial suspension and reduction in absorbance at 490nm was determined after 0.5 and 4.5min of incubations at 25 °C using a microplate reader. One unit of lysozyme activity was defined as the amount of enzyme causing a decrease in absorbance of 0.001 per min.
Statistical analysis
All results for each parameter measured were expressed as means±standard errors, and were compared at each time point using Student's t-test for independent data. Significant differences between experimental groups were expressed at a significance level of p <0.05. All analyses were carried out on 15 fish per group.
Results
Hematological analysis
Dietary Origanum vulgare extract incorporated test diets had no significant (p <0.05) effect on red blood cell count (RBC), white blood cell count (WBC), differential leukocytes count (monocyte, lymphocyte and neutrophile), hematocrit (Hct), hemoglobin (Hb), the all the values of red blood cell indices, the mean values of cell hemoglobin (MCH pg), cell hemoglobin concentration (MCHC %), and cell hemoglobin volume (MCV fl) at the end of none of the identical two weeks after feeding in compared to placebo group (Table 1).
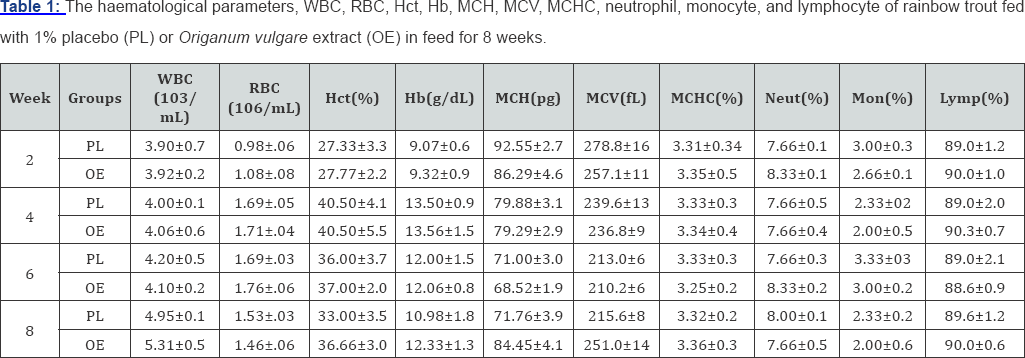
Data are expressed as mean±SE (n=15). No significant differences were observed in the Origanum vulgare treated groups relative to the placebo group at the end of the identical every two weeks after feeding (P>0.05). Neut: neutrophil; Mon: Monocyte; Lymp: Lymphocyte.
Biochemical analysis
Origanum vulgare extract had significant (P<0.05) effect in increase of total protein (TP), albumin (AL), and globulin (GL), at the end of the identical every two weeks after feeding in compared to placebo group (Table 2). The maximum level of total protein was recorded on week 2 of exposure duration. Similarly, albumin and globulin contents were significantly higher in Aloe vera group as compared to placebo group. However, albumin/globulin ratio was not exhibited significant differences in two weeks after feeding in compared to placebo group (p>0.05; compared to placebo group at the end of the identical every Table 2).
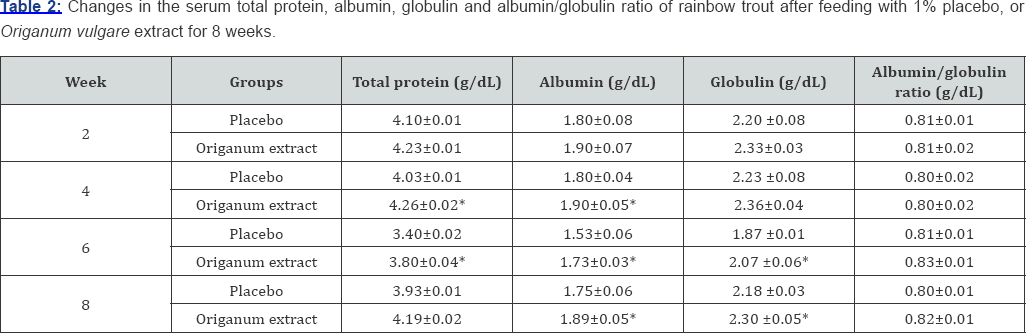
Data are expressed as mean±SE (n=15). *: P<0.05 compared with the
Immunological analysis
Respiratory burst activity

The respiratory burst activity significantly (P<0.05) enhanced in fish fed with 1% of Origanum vulgare extract supplementation feed at the end of the identical every two weeks after feeding in compared to placebo group (Figure 1).
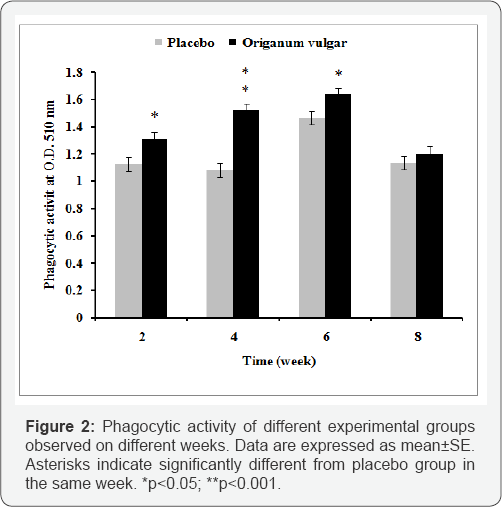
Phagocytic activity of blood leucocytes significantly (P<0.05) enhanced in fish treated with 1% of Origanum vulgare extract supplementation feed at the end of the identical every two weeks after feeding in compared to placebo group (Figure 2).
Lysozyme activity
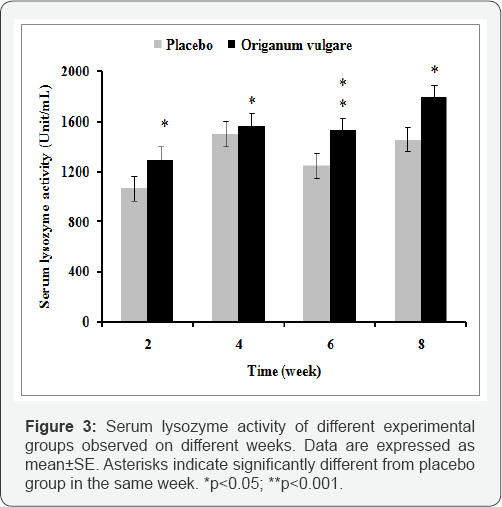
Lysozyme activity significantly (P<0.05) enhanced in fish fed with 1% of Origanum vulgare extract supplementation feed at the end of the identical every two weeks after feeding in compared to placebo group (Figure 3).
Discussion
The present study projects the impact of dried Origanum vulgare extract on the hematological and immunological responses in rainbow trout (Oncorhyncous mykiss). The hematological parameters in the present investigation such as RBC, WBC, differential leukocytes counts, hemoglobin, hematocrit, the all of the values of red blood cell indices (MCH, MCHC and MCV) were no significant differences at the end of none of the identical every two weeks after feeding when compared to control group. These observations are in agreement with the obtained results of other researchers, who reported that rainbow trout treated with dietary Aloe vera supplementation were no significant differences in RBC and Hct [18], or RBC and Hb [19] among the groups.
In the present study, the dietary Origanum vulgare extract supplementation enhanced total plasma protein, albumin and globulin values in comparison with control group. Similar results were reported in rainbow trout fed with garlic [20], ginger [21], lipopolysaccharide [22], Laurus nobilis [23], and Coggyria coggyria [24]. Serum proteins are various humoral elements of the non-specific immune system, measurable total protein, albumin and globulin levels suggest that high concentrations are likely to be a result of the enhancement of the non-specific immune response of fish. So, this study revealed that Origanum vulgare extracts incorporated diets helped to increase the humoral elements in the serum. Respiratory burst activity is considered as an important indicator of non-specific defense in fish, which is a measure of the increase of oxidation level in phagocytes stimulated by foreign agents [25]. An enhancement of respiratory burst activity has been identified in the present study, that it is in agreement many of studies with dietary immunostimulants [23,26]. Respiratory burst and phagocytosis response by phagocytes in blood present a major antibacterial defense mechanism in fish [27]. Phagocytosis is one of the most important processes in fish. The main cells involved in phagocytosis in fish are neutrophils and macrophages. These cells remove bacteria mainly by the production of reactive oxygen species (ROS) during a respiratory burst. In addition, neutrophils possess myeloperoxidase in their cytoplasmic granules, which in the presence of halide and hydrogen peroxidase kills bacteria by halogenations of the bacterial cell wall. Moreover, these cells have lysozymes and other hydrolytic enzymes in their lysosomes [28]. Similarly, macrophages can produce nitric oxide in mammals and can be as potent as antibacterial agents, peroxynitrates and hydroxyl groups. Phagocytic activity of leucocytes in rainbow trout was enhanced by dietary dose of powdered ginger rhizome [29,30]. Also, in this study an increasing trend in lysozyme activity has been shown which is in agreement with several reports indicating the role of herbal immunostimulants in enhancing lysozyme activity [31-33]. Lysozyme is a humoral component of the non-specific defense mechanism which has the ability to prevent the growth of bacteria by splitting p-1,4 glycosidic bonds in the peptidoglycan of bacterial cell walls, resulting in bacteriolysis. In conclusion, supplementation of OE in aquaculture diets would be use to enhance non-specific immune system in fish. Therefore, further studies are necessary for effective use of Origanum vulgare extract with optimal dose, suitable duration, and method of administration.
Comments
Post a Comment