Rendez-Vous at the Baltic? The Ongoing Dispersion of the Black-Striped Pipefish, Syngnathus abaster- Juniper Publishers
Juniper Publishers- Journal of Oceanography
Abstract
Syngnathus abaster is a pipefish whose distribution is commonly referred as encompassing the Mediterranean and Black Sea, as well as the Atlantic Coast northwards up to the Bay of Biscay. Given the species ability to endure large variation in water salinity, this euryhaline pipefish may now be encountered in a wider variety of aquatic environments, not only marine or brackish but also in purely freshwater habitats. During the last decades, information on the biology of the black-striped pipefish grew, and new data emerged. As this information derives from populations that are geographically distant, it is important to review the available data across the distribution in order to understand the more general processes occurring. While summarizing the distributional information, collected data suggests that S. abaster is expanding its spatial distribution in two very distinct ways, either by slowly moving north through the Eastern Atlantic Coast, or by successively establishing viable populations in freshwater ecosystems, in the east. Available data on reproduction suggests that all the sampled populations reproduce at roughly identical temperature ranges, advocating that this species is mainly a spring and summer spawner, at least when inhabiting marine environments. Nevertheless, important inter-populational differences exist in several reproduction-related variables, such as the maximum egg number carried by pregnant males, hinting that the current taxonomy is in need of an extended revision. Open questions related to the causes and implications of freshwater colonization are also further discussed.
Keywords: Syngnathus abaster; Distribution; Reproduction; Freshwater expansion; Breeding season
Introduction
The black-striped pipefish, Syngnathus abaster [1], is a small brown-green syngnathid, with dark or pale spots on the trunk and tail. Adults of both sexes can be easily distinguished by the presence of an inverted brood pouch (marsupium) located in the tail, formed by two skin-folds that contact medially with their free edges. Females lay eggs into the marsupium, where they are fertilised and incubated for about 20-30 days, depending on water temperature. The biogeographic distribution of this pipefish is commonly referred as encompassing the Mediterranean and Black Seas, as well as the Atlantic Coast northwards up to southern Biscay [2]. Nevertheless, given the species ability to endure a large range of salinities, this euryhaline pipefish may now be encountered in a wider variety of aquatic environments, not only marine or brackish but in purely freshwater habitats. In fact, during the last few decades, stable populations of S. abaster have been reported inhabiting rivers, such as the Danube, more than 900km away from the river mouth [3,4]. Interestingly, on the other side of the salinity spectrum, it is also common to observe the black-striped pipefish breeding in reservoirs traditionally used to concentrate water that will ultimately feed salt pans. Altogether, these observations highlight the need for a more up-to-date representation of the species geographical distribution, merging sightings from coastal sites together with the most recent data on freshwater colonisations.
At a micro-geographical scale, at least in coastal areas, S. abaster can be found among vegetation, detritus or even sand or mud substrates [2,5]. Nevertheless, a clear preference for sea grass meadows has been generally reported [6-8] where this pipefish tends to occupy a position close to the bottom [6]. In this type of microhabitat, S. abaster feeds on small preys hidden in the vegetation, sucking them through its cone shaped snout [9-12]. The presence of this pipefish in freshwater habitats seems also to be closely related with vegetated areas, where it tends to occupy the lower part of the channels [13]. Although the conservation status of S. abaster is currently catalogued as "Least Concern", according to the IUCN Red List of Threatened Species [14], the close relationship with vegetation, namely sea grass, might explain the steep population decline that can be observed in estuarine areas which are increasingly subjected to strong anthropogenic pressure [15,16].
During the last decade, information on the biology of the black-striped pipefish grew, and new data on the biology of the species emerged. The courtship and mating ritual has been described [17] showing that the general behavioral patterns seem reasonably conserved within the Syngnathidae. Also, the embryonic and larval development was characterized, highlighting the fact that newborn juveniles immediately adopt a benthic distribution [18]. The absence of a pelagic life phase probably has important repercussions in terms of population connectivity given the ongoing fragmentation and degradation of the preferred eelgrass habitat.
A closer analysis of S. abaster mating dynamics revealed that this sex-role reversed pipefish tends to mate size-assortatively [19] and that male brooding space did not appear to be the only limitation to female reproduction as neither large nor small individuals presented a fully occupied pouch during the breeding season [20]. Besides revealing a multiple mating strategy and the fact that females do not seem to produce enough eggs to fully occupy a male's brood pouch during the extent of a pregnancy, [21,22] also showed that different-sized individuals adopt different investment tactics, through a continuous scan of the social environment. Temperature fluctuations seem also to play an important role in S. abaster reproduction, acting as an effective agent in the modulation of the reproductive behavior, directly affecting sexual recognition, mate preferences and female-female interactions [23]. Given the current climatic changes, the local expression of a species mating system will surely vary [24], with consequences that cannot yet be foreseen given the small amount of information available on estuarine, coastal and freshwater populations.
Although information on S. abaster life history is growing, it is frequently sparse and often times superficial, namely that related to the species life span or reproductive ecology. As an example, for a population inhabiting the brackish bay of the Po river delta [9], authors predicts a life span of approximately 17 months. Full life-cycle aquarium experiments (N. Monteiro, personal observation), conducted with individuals from the Portuguese coast, suggest that the values presented by Freyhof & Kottelat [25], which mention a life span up to 4 years, might be much more realistic. Large discrepancies exist also on the number of eggs that a male can carry during a pregnancy event. Franzoi et al. [9]. show that males carry on average 109 eggs while data from Atlantic populations reveals a much smaller number of potential offspring (e.g. 69 in the Tagus Estuary). As this information derives from populations that are geographically distant, it seems crucial to review the available data from all across the species distribution, in order to extend our knowledge on the more general processes that might be occurring. This approach could not only allows for a better understanding on general trends versus local adaptations, but also generates a predictive potential able to assess ongoing or future alterations under a climate change scenario. To this extent, this work attempts to summarize the information on the biogeography of the black-striped pipefish, and further extend our knowledge on the species distribution by including the most recent recordings, namely in the European Atlantic coast, as well as in recently colonized freshwater habitats.
Materials and Methods
Information on the biogeographic distribution of Syngnathus abaster was collected from numerous different source types. Basically, the data presented by Dawson [2] was complemented with information provided by Freyhof & Kottelat [25]. The distributional area was further extended by the addition of recordings from Belarus [26], the Atlantic coast of France (GBIF Data Portal, data.gbif.org) and Germany (sesam.senckenberg. de). Additionally, apart from the above-mentioned sources, a bibliographic survey was conducted in order to gather site- specific information on the presence of S. abaster and, when possible, data on the species reproductive season and migratory vs. resident behavior. Given that this species has numerous synonyms, some still recurrently found in the literature, the survey was conducted also using the terms S. algeriensis, S. agassizii or S. nigrolineatus.The summary of the obtained data was plotted in, based on the following information (here discriminated by country): Portugal [27-32]; Spain [33,34]; France [35-37]; Italy [5,6,8-10,38-43]; Croatia [44]; Serbia [4]; Greece [38,45,46]; Turkey [47,48]; Bulgaria [49]; Ukraine [4,13,50]; Belarus [26]; Russian Federation [13,51,52]; Iran [5355]; Egypt [38]; Tunisia [1,56]; and Algeria [57].
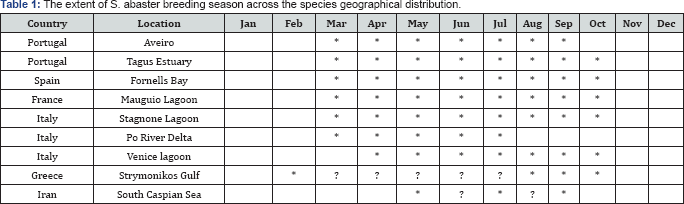
In order to construct a more general view of S. abaster reproductive season across the species' geographical distribution see Table 1, the information available in the literature was used Portugal [27]; Spain [33]; France [36]; Italy [9,10,41]; Greece [45,54]. New data is provided for Aveiro, Portugal (40° 38’N, 8°39'W).
Results
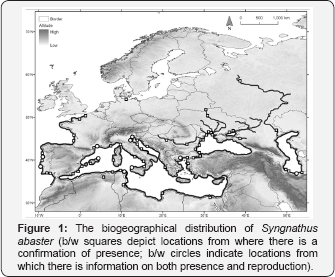
What seems to be the most up-to-date map of Syngnathus abaster distribution is depicted in Figure 1. The distribution presented by [2] had already been considerable extended by[25] , mainly by the inclusion of the Caspian Sea, Sea of Azov, and freshwater locations (e.g. Danube, Dnieper, Don and Volga). The presence of the black-striped pipefish in these locations was confirmed, following a literature review, . Additionally, the presence in Rybinskoye Reservoir (Russia) was also validated through an in-situ observation (S. Drovetsky, personal observation). A northwards colonization of the Dnieper seems to be occurring, as S. abaster is now present in Belarus)[26] . In the European Atlantic Coast, new observations exist northwards of Biscay, the reported northern limit of distribution in the Atlantic. These new locations, both in France and Germany, if further validated through repeated observations, suggest a rapid colonisation of the Atlantic up to the Baltic Sea.
In Aveiro (Portugal), the breeding season of S. abaster occurs from March to September [19]. Sampling campaigns conducted outside the breeding season, that included the trawling of several locations within this estuarine lagoon, did not allow the capture of any individual. These observations suggest that this pipefish uses this specific location as a reproduction area, abandoning the area at the end of the breeding season. This data, when integrated with that already available for other locations (see Figure 1) suggests that the breeding season occurs generally from March to October (Table 1), probably dependent on the warmer water temperatures.
When looking at the maximum number of eggs present in pregnant males, large discrepancies are observed. While in Portugal (53 eggs recorded during the 2010 field season) and France 52 eggs; [36] the reported values are similar, the maximum number of eggs reported for the Italian populations, in the Adriatic area [9,41], are clearly much higher (Venice, 122 eggs; Po River Delta, 136 eggs).
Discussion
Syngnathus abaster has been traditionally viewed as a Mediterranean pipefish, as the main bulks of observations were concentrated in this area, although it has been reported also in the Black Sea and throughout the Iberian Peninsula Coast [see 2]. More recent data, however, suggest that this species has greatly increased its distribution, in two very distinct manners occurring in geographically opposite locations:
-Firstly, by expanding its northern limit of distribution in the Atlantic coast, surpassing the Bay of Biscay, towards the Baltic Sea. If the collected observations are further corroborated by additional captures, it suggests a pattern of colonization consistent with what is currently being observed with other warm-water species whose northern limit of distribution is moving polewards [58-60], probably as a consequence of climate change see [61]. If this rapid colonization of the Atlantic is indeed taking place, as evidence suggests, the monitoring of the reproductive ecology of Atlantic populations gains a renewed importance as it can potentially provide new insights on previously undescribed adaptive responses exhibited by this pipefish.
-And secondly, by establishing viable populations in freshwater ecosystems in the eastern part of the species' distribution. A closer inspection of Figure 1 immediately generates an inescapable question: Why has this freshwater colonisation occurred only in the eastern part of the distribution, when there are rivers in the West as well? The simplest explanation to this phenomenon might just be that this freshwater invasion is a result of anthropogenic action rather than an active migration performed by this pipefish. If fish indeed actively colonised freshwater environments, there were no obvious reasons that obstructed their presence in rivers that flowed into more western sections of the Mediterranean. If, on the other hand, S. abaster, is being introduced into freshwater ecosystem, then it could be hypothesised that rivers large enough to harbour ship traffic are more prone to receive these guests from marine or brackish areas. With the exception of the Rhone, large enough rivers able to accommodate high levels of traffic (such as the Loire, Seine, Loire or Rhine) do not flow into the Mediterranean in its western section. Such rivers do exist in the east, however, and they all seem to be recently colonized by S. abaster (e.g. Danube, Dnieper, Don or Volga). Indeed, Semenchenko & Cakic et al. [4,26] state that commercial ships acted as vectors, probably through ship ballast-water. The presence of the black-striped pipefish in water reservoirs could also be due to accidental introductions, as seems to have occurred in middle and lower Volga region, where these fish where brought together with mysids from the Don estuary [52]. The existence of dams, for instance, probably eased the establishment of freshwater S. abaster populations by creating more favourable hydrodynamic conditions.
The Mediterranean populations from where data exists on the timing of the breeding season all seem to reproduce at roughly identical temperature ranges, suggesting that this species is mainly a spring and summer spawner. But, when observing the distributional data, it seems obvious that freshwater populations are subjected to very distinct temperature regimes. This fact raises several interesting questions, such as when and how breeding takes place in these cooler locations, thus reinforcing the need for more complete sampling campaigns all over the species distribution [62].
Even within the Mediterranean basin, there seem to exist important differences among populations in several reproduction related variables, either behavioral (there are resident populations that inhabit a certain location throughout the year while in other places individuals migrate to coastal areas to breed) or morphological/physiological (the maximum number of eggs that is dependent on either male/marsupium size, egg size or a combination of both, greatly varies in certain locations). A thorough molecular analysis will surely help answer many of the questions that still persist such as the most basic one: are we really talking about just one species.
To Know More About Journal of Oceanography Please Click on: https://juniperpublishers.com/ofoaj/index.php
To Know More About Open Access Journals Publishers Please Click on: Juniper Publishers
Comments
Post a Comment