Melanized Lesions in Red Swamp Crayfish (Procambarus clarkii) Reared in the Laboratory Conditions
Oceanography & Fisheries Open Access Journal Juniper Publishers
Abstract
Red swamp crayfish (Procambarus clarkii) is an economic decapod species and the most cultivated crayfish in the world. In nature, this species can tolerate the hardy alterations, but they are vulnerable to water parameters in culture conditions. Crayfish have to molt to grow, and the non-renewable shell is a suitable surface for pathogenic activities until the next molt. In this study, melanized lesions were determined in red swamp crayfish from several parts of body exoskeleton: cephalothorax, abdominal shell, uropods, walking legs, swimmerets, and antennae. The bacteriological and mycological isolations were conducted onto tryptic soy agar, brain heart infusion agar, Sabouraud’s dextrose agar and glucose yeast agar, but no particular microbial growth was observed from cultured samples. The aetiology of the case was not associated with any bacterial or fungal agents; however, the water conditions could be related with the melanosis.
Keywords: Cambaridae; Molting; Shell rot; Exoskeleton erosion; Appendages
Introduction
Red swamp crayfish (Procambarus clarkii) is native from north-eastern Mexico and United States (Louisiana). The species is resistant to extreme conditions, including oxygen and temperature alterations, water pollution, and drought and located in wetlands, marshes, rivers, and lakes [1,2]. However, this crayfish is the most cultivated species in the world and has an economic importance for both aquaculture and aquarium sectors [3]. Besides the popularity of this species, invasive effects have been recorded from North and South America, Europe, Asia, and Africa [4]. In Turkey, no commercial red swamp crayfish cultivation has been done. This species accepted as a pet in the last quarter of the 2000s and traded as the most common crayfish species in the aquarium industry of Turkey. Red swamp crayfish is a member of Order Decapoda (Crustacea), which characterized by ten jointed legs and other appendages. Five pairs of pereiopods (walking legs) and pleopods (swimming legs) exist beneath their cephalothorax and abdomen regions [5]. Segmented antennas and antennules are vulnerable against the physical attacks. Crayfish have to molt to grow, and the molting frequency decreases as the crayfish grows [6]. This causes microorganisms that adhere to these appendages and body regions that cannot be regenerated (until the next molt) to stay longer and increase the pathogenic potential. There are several pathogens that cause disease in crayfish; Saprolegnia parasitica, Trichosporon jirovecii, Vibrio species [7-9], and melanized lesions are very common in many cases. Despite tolerating the wide range of water parameters, the relevant stress cause pathological lesions on the exoskeleton of crayfish. In this case, pre-identification study on tissue samples from decaying body parts and appendages of laboratory-reared red swamp crayfish was reported.
Materials and Methods
Laboratory Conditions and Rearing System
The case was observed in Tropical Aquaculture Laboratory, Faculty of Fisheries, İzmir Kâtip Çelebi University, Çiğli, İzmir, Turkey. Red swamp crayfish (Procambarus clarkii) were obtained from a commercial facility and reared in the laboratory conditions. The crayfish were stocked into two recirculating freshwater sump systems (270 L in each) and fed twice daily with commercial bottom feed (Artakua®, Tire, İzmir, Turkey), routinely. In the sump system, a submersible pump (Aquawing AQ6000) transfers water to the plastic containers (10 L) and U-PVC outlines collect the water to the glass sump aquarium (150 L). System water is filtered by passing through the biological sponge and resting with activated carbon, ceramics, and bio-balls. The filtered water is transferred back to the containers with the submersible pump.
Water Parameters
The temperature in the systems was held at 25-26 °C with external heaters (Hydor ETH 300). Dissolved oxygen and pH were measured daily with AZ 84051 Combo Water Meter and their range were recorded as 8.70-9.50 ppm and 7.30-7.80, respectively.
Microbiological Tests
The crayfish showing melanized lesions on different the parts of the exoskeleton were sampled (n=10) and microbiological studies were conducted in the Fish Disease and Biotechnology Laboratory, Faculty of Fisheries, İzmir Katip Celebi University, Çiğli, İzmir, Turkey. The specimens were examined in detail and the hemolymph samples were streaked onto tryptic soy agar (TSA, Merck) and brain heart infusion agar (BHIA, Merck) for any bacterial infection. Samples from melanized lesions were cultured onto Sabouraud’s dextrose agar (SDA, Merck) and glucose yeast agar (GYA) for the fungus culture. The plates were incubated at 25 °C and regularly checked for bacterial or fungal growth.
Results
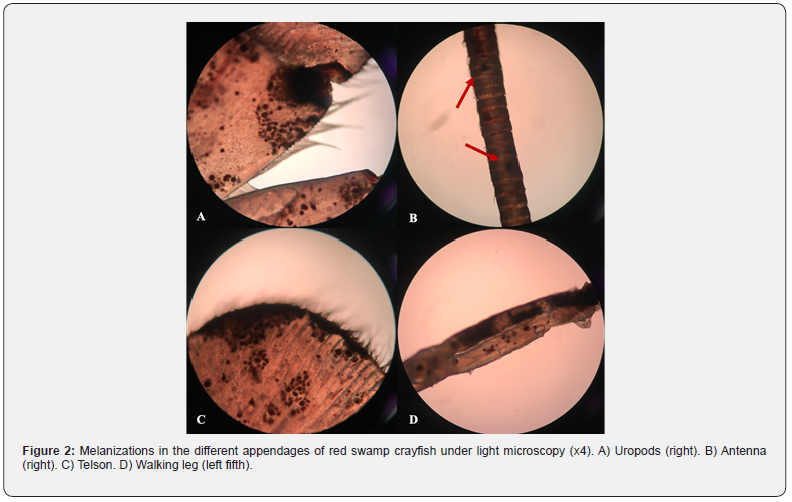
The melanosis and erosions on the exoskeleton of crayfish were determined especially in body regions (cephalothorax, abdomen, telson, and uropods) and appendages (walking legs, swimmerets, chelipeds, joints, antennae, and antennules) (Figure 1). These body parts examined under the light microscopy (Olympus BX53) and intense melanizations were observed (Figure 2). A causative pathogen wasn’t isolated from diseased individuals. A causative pathogen wasn’t isolated from diseased individuals.

Discussion
Salighehzadeh et al. [10] reported melanized abdominal lesions in narrow-clawed crayfish (Astacus leptodactylus) and determined co-infection caused by Aeromonas hydrophila and Fusairum solani. Findings obtained from the microbiological tests were not compromised with the current study although applying the same methodology. Similarly, Abdallah et al. [8] isolated Trichosporon jirovecii from the melanized exoskeleton of red swamp crayfish (Procambarus clarkii) collected from the River Nile. The isolation and purification of the fungi were performed onto GYA and SDA from melanized uropod, walking legs, abdominal shell, telson, swimmerets, rostrum and antennae. Moreover, complete loss of uropods, walking legs, swimmerets, telsons and / or antennae were recorded in some cases. Krugner-Higby et al. [11] monitored native crayfish (Orconectes propinquus) with ulcerative lesions on the carapace and legs from Big Muskellunge Lake (USA). The lesions were reported discolored as brownishblack spots because of melanin deposition linked to Saprolegnia australis infection.
Likewise, there are several reports of idiopathic conditions of Australian red claw crayfish (Cherax quadricarinatus) just as black to dark, blue-colored spots on the exoskeleton [12-15]. Edgerton [14] observed round, dark blue to black spots in the exoskeleton of C. quadriacarinatus ‘pin-prick’ pit at the center of the spot. The melanization in the cuticle was associated with abiotic and biotic irritants. Moreover, nutritional factors were also mentioned to be considered in future studies. In this study, the bacteriology and mycology studies were not present any results that support infectious causes of this syndrome despite using the same methodology with similar research.
Conclusion
In conclusion, further detailed studies are necessary to determine the causative agents of the disease. The melanosis on the exoskeleton of crayfish is become a limiting factor for aquarium sector that cause economic loss.
To Know More About Oceanography & Fisheries Open Access Journal Please click on:
For more Open Access Journals in Juniper Publishers please click on:

Comments
Post a Comment