SDS-PAGE Electrophoresis of Ocypode rotundata Miers, 1882 and O. ceratophthalma Pallas, 1772 using Muscle Tissue along the Coast of Pakistan
Oceanography & Fisheries Open Access Journal Juniper Publishers
Authored by Sahir Odhano
Abstract
Electrophoretic studies for the identification of ghost crabs (Ocypode rotundata and Ocypode ceratophthalma) using
the SDS-PAGE were performed on protein patterns along the Sandspit
coastal areas. The muscle tissues were used to estimate the molecular
mass of proteins through SDS-PAGE electrophoresis. Electrophoretic
settings were standardized to use 10% acrylamide resolving gel and 5%
acrylamide stacking gel. A discontinuous buffer system was used
following the protocols of Laemmli (1970) to observe the relative
mobility and molecular weight of proteins. In O. rotundata total
of 6 protein bands were resolved among them band-5 was found similar in
size to the marker used (BSA = 66 kDa MW). Whereas in O. ceratophthalma total
of 8 protein bands were resolved among them three protein bands
(band-6, band-7, and band-8) were found to be higher in size than the
molecular marker. Both species revealed four protein bands smaller than
BSA ranging from 20 kDa to 40 kDa MW. The smaller-sized protein bands
were suspected to be myosin light chain protein bands, sarcoplasmic
calcium-binding proteins (SCBP), tropomyosin, and arginine kinase.
Results showed that both species of ghost crabs are distinct from each
other through their banding pattern and their relative mobility. The
current study revealed the possible efficacy of SDS-PAGE for the
identification of ghost crab species by using the standard molecular
marker. However, few molecular proteins may also be used as markers for
species identification. Therefore, SDS-PAGE may also be used for
species-specific protein identification and linked to immune-analytic
techniques.
Keywords: Muscle Tissue, Ocypode; O. rotundata; O. ceratophthalma; Ghost crabs; SDS-PAGE; BSA; Sandspit, Karachi
Electrophoretic studies for the identification of ghost crabs (Ocypode rotundata and Ocypode ceratophthalma) using the SDS-PAGE were performed on protein patterns along the Sandspit coastal areas. The muscle tissues were used to estimate the molecular mass of proteins through SDS-PAGE electrophoresis. Electrophoretic settings were standardized to use 10% acrylamide resolving gel and 5% acrylamide stacking gel. A discontinuous buffer system was used following the protocols of Laemmli (1970) to observe the relative mobility and molecular weight of proteins. In O. rotundata total of 6 protein bands were resolved among them band-5 was found similar in size to the marker used (BSA = 66 kDa MW). Whereas in O. ceratophthalma total of 8 protein bands were resolved among them three protein bands (band-6, band-7, and band-8) were found to be higher in size than the molecular marker. Both species revealed four protein bands smaller than BSA ranging from 20 kDa to 40 kDa MW. The smaller-sized protein bands were suspected to be myosin light chain protein bands, sarcoplasmic calcium-binding proteins (SCBP), tropomyosin, and arginine kinase. Results showed that both species of ghost crabs are distinct from each other through their banding pattern and their relative mobility. The current study revealed the possible efficacy of SDS-PAGE for the identification of ghost crab species by using the standard molecular marker. However, few molecular proteins may also be used as markers for species identification. Therefore, SDS-PAGE may also be used for species-specific protein identification and linked to immune-analytic techniques.
Keywords: Muscle Tissue, Ocypode; O. rotundata; O. ceratophthalma; Ghost crabs; SDS-PAGE; BSA; Sandspit, Karachi
Introduction
Ghost crabs are the most active, swift running, and
aerobically fit crustacean species. These crabs are semi-terrestrial
species and known as the fastest running invertebrates. They belong to
the genus Ocypode Weber, 1795 (‘swift-footed’) family Ocypodidae.
Their body muscles allow them to run swiftly at the maximum speed of
3-4 m/s [1-3] and can walk slowly and continuously for up to an hour
[4]. There are two types of skeletal muscles involved in powering the
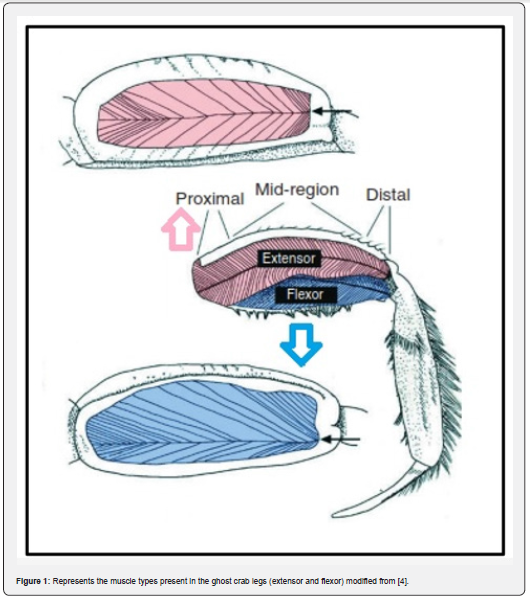
crabs for fast running the extensors and flexors [4] (Figure 1).
According to Priya et al. [3] these muscles receive enough supply of O2
through hemolymph for their running capabilities. The fiddler crabs
also belong to the same family, but they are not capable of running too
fast as compared to the ghost crabs.
Despite obvious differences between ghost crabs and
other running vertebrates, the biochemical study revealed that the ghost
crabs interestingly share many features of locomotion with other
running vertebrates such as similar stride frequencies and trot to
gallop transition [4,5]. The O2 supply to skeletal muscles
occurs through the cardiovascular system to generate more energy for
swift running. The physiological adaption for such a mechanism to
support sustainable muscle power is partially known. Weibel &
Hoppeler [6] studied vertebrate skeletal muscles and described the
strong correlation between the aerobic capacity of whole animals and
the volume of mitochondria within the skeletal muscles. There are many
crustaceans who are active and highly alert due to the efficient supply
of O2 to their skeletal muscles. But very little is known about skeletal muscle mechanism in invertebrates.
Electrophoretic studies are a widely used technique in tissues
for protein analysis. Muscle tissues are the most suitable
part for such a study to separate various peptide bands through
their weight. Sodium Dodecyl Sulphate (SDS) is usually used in
polyacrylamide gel electrophoresis (PAGE) to separate the protein
according to their size and weight [7,8]. Therefore, SDS-PAGE is a
technique used to isolate each peptide band, when current is applied
and each band is observed in the gel [3,8-11].
Davis (1964) reported the method for separating serum proteins
using polyacrylamide gel electrophoresis, and a significant
advancement is observed in protein electrophoresis subsequently.
Myofibril proteins are insoluble in PAGE electrophoresis and do
not produce any results, therefore SDS was introduced in tissue
sample and gel solution. This new approach helped the investigators
resolve the issue and gave effective results [12,13]. Therefore,
nowadays, SDS-PAGE is a simple technique to easily separate
proteins through their molecular weight. SDS-PAGE is the most
efficient, cost-effective technique, typically used to identify the
molecular weight of proteins. SDS neutralizes the protein segment
and denature it to estimate the protein weight easily
Ghost crabs are the most active, swift running, and aerobically fit crustacean species. These crabs are semi-terrestrial species and known as the fastest running invertebrates. They belong to the genus Ocypode Weber, 1795 (‘swift-footed’) family Ocypodidae. Their body muscles allow them to run swiftly at the maximum speed of 3-4 m/s [1-3] and can walk slowly and continuously for up to an hour [4]. There are two types of skeletal muscles involved in powering the crabs for fast running the extensors and flexors [4] (Figure 1). According to Priya et al. [3] these muscles receive enough supply of O2 through hemolymph for their running capabilities. The fiddler crabs also belong to the same family, but they are not capable of running too fast as compared to the ghost crabs.
Despite obvious differences between ghost crabs and other running vertebrates, the biochemical study revealed that the ghost crabs interestingly share many features of locomotion with other running vertebrates such as similar stride frequencies and trot to gallop transition [4,5]. The O2 supply to skeletal muscles occurs through the cardiovascular system to generate more energy for swift running. The physiological adaption for such a mechanism to support sustainable muscle power is partially known. Weibel & Hoppeler [6] studied vertebrate skeletal muscles and described the strong correlation between the aerobic capacity of whole animals and the volume of mitochondria within the skeletal muscles. There are many crustaceans who are active and highly alert due to the efficient supply of O2 to their skeletal muscles. But very little is known about skeletal muscle mechanism in invertebrates.
Electrophoretic studies are a widely used technique in tissues for protein analysis. Muscle tissues are the most suitable part for such a study to separate various peptide bands through their weight. Sodium Dodecyl Sulphate (SDS) is usually used in polyacrylamide gel electrophoresis (PAGE) to separate the protein according to their size and weight [7,8]. Therefore, SDS-PAGE is a technique used to isolate each peptide band, when current is applied and each band is observed in the gel [3,8-11].

Davis (1964) reported the method for separating serum proteins using polyacrylamide gel electrophoresis, and a significant advancement is observed in protein electrophoresis subsequently. Myofibril proteins are insoluble in PAGE electrophoresis and do not produce any results, therefore SDS was introduced in tissue sample and gel solution. This new approach helped the investigators resolve the issue and gave effective results [12,13]. Therefore, nowadays, SDS-PAGE is a simple technique to easily separate proteins through their molecular weight. SDS-PAGE is the most efficient, cost-effective technique, typically used to identify the molecular weight of proteins. SDS neutralizes the protein segment and denature it to estimate the protein weight easily
Materials and Methods
Study area
The specimens were collected randomly from the
Sandspit (24.84, 66.91) sandy coastal area from 2014-2015 at the low
tide. All the specimens were placed in tagged polyethene bags and
brought to laboratory for electrophoretic study. Each specimen was
sorted out through taxonomic identification keys [14,15]. O. rotundata specimen and O. ceratophthalma specimen were placed separately for muscle tissue extraction.
The specimens were collected randomly from the Sandspit (24.84, 66.91) sandy coastal area from 2014-2015 at the low tide. All the specimens were placed in tagged polyethene bags and brought to laboratory for electrophoretic study. Each specimen was sorted out through taxonomic identification keys [14,15]. O. rotundata specimen and O. ceratophthalma specimen were placed separately for muscle tissue extraction.
Tissue extraction
The muscle tissue sample was extracted (approx. 1g) from
the enlarged cheliped of each individual species. The tissue sample
was ground in a hand-held homogenizer in extraction buffer
(Tris–citrate, 0.687M Tris and 0.157M Citrate pH 8.0) to homogenize
the tissue prior to electrophoresis. The homogenized sample
was centrifuged at 14500 rpm and the supernatant was poured
into a new well-labelled Eppendorf tube for gel electrophoresis.
The muscle tissue sample was extracted (approx. 1g) from the enlarged cheliped of each individual species. The tissue sample was ground in a hand-held homogenizer in extraction buffer (Tris–citrate, 0.687M Tris and 0.157M Citrate pH 8.0) to homogenize the tissue prior to electrophoresis. The homogenized sample was centrifuged at 14500 rpm and the supernatant was poured into a new well-labelled Eppendorf tube for gel electrophoresis.
Solution preparation
The proteins present in tissue sample (Supernatant) were
separated by SDS-PAGE vertical slab gel following the Laemmle
(1970) protocols. A 10% acrylamide running gel (resolving) was
prepared by using Tris-HCL buffer (pH 8.8), whereas a 5% spacer
gel (stacking gel) was prepared by using Tris-HCL buffer (pH 6.8).
Tris-glycine buffer (pH 8.5) was prepared as Tank buffer. The muscle
tissue and A mixture of glycerol, tris-HCl buffer (pH 6.8), 10%
SDS, and mercaptoethanol were homogenized through a centrifuge
machine at 14500 rpm for the protein extraction.
The proteins present in tissue sample (Supernatant) were separated by SDS-PAGE vertical slab gel following the Laemmle (1970) protocols. A 10% acrylamide running gel (resolving) was prepared by using Tris-HCL buffer (pH 8.8), whereas a 5% spacer gel (stacking gel) was prepared by using Tris-HCL buffer (pH 6.8). Tris-glycine buffer (pH 8.5) was prepared as Tank buffer. The muscle tissue and A mixture of glycerol, tris-HCl buffer (pH 6.8), 10% SDS, and mercaptoethanol were homogenized through a centrifuge machine at 14500 rpm for the protein extraction.
Gel assembly casting
The glass plates of the electrophoresis apparatus were washed
and carefully cleaned with ethanol and were fixed on the assembly
using the spacers and clips. A resolving gel was prepared in a 50ml
beaker ((3.2ml distilled water, 2.64ml 30% acrylamide, 2ml 1.5
M Tris, 0.5ml 10% SDS, 1ml 10% Ammonium persulfate (APS),
0.5μlml N, N, N, N N”-Tri methylamine triamine (TEMED) and the
solution was poured into gel plate and waited for the gel to get polymerized
(maximum 30 minutes). The resolving gel was poured about 2/3rd area of the gel plate and the rest of the area was filled with distilled water to level the resolving gel. When the resolving
gel got polymerized and then distilled water was taken off and the
stacking gel was prepared. The stacking gel was formed in a beaker
(2.1ml distilled water, 0.5ml 30% acrylamide, 0.38ml of 1.0M
Tris, 0.03ml 10% SDS, 0.03ml 10% APS, 0.003ml TEMED) the APS
and TEMED were added just before pouring the upper cast of
gel plates. Immediately the gel comb was inserted before the gel
polymerized. After polymerization, the comb was removed, and
wells were washed with distilled water.
The glass plates of the electrophoresis apparatus were washed and carefully cleaned with ethanol and were fixed on the assembly using the spacers and clips. A resolving gel was prepared in a 50ml beaker ((3.2ml distilled water, 2.64ml 30% acrylamide, 2ml 1.5 M Tris, 0.5ml 10% SDS, 1ml 10% Ammonium persulfate (APS), 0.5μlml N, N, N, N N”-Tri methylamine triamine (TEMED) and the solution was poured into gel plate and waited for the gel to get polymerized (maximum 30 minutes). The resolving gel was poured about 2/3rd area of the gel plate and the rest of the area was filled with distilled water to level the resolving gel. When the resolving gel got polymerized and then distilled water was taken off and the stacking gel was prepared. The stacking gel was formed in a beaker (2.1ml distilled water, 0.5ml 30% acrylamide, 0.38ml of 1.0M Tris, 0.03ml 10% SDS, 0.03ml 10% APS, 0.003ml TEMED) the APS and TEMED were added just before pouring the upper cast of gel plates. Immediately the gel comb was inserted before the gel polymerized. After polymerization, the comb was removed, and wells were washed with distilled water.
Sample loading, staining and de-staining
A 20-μl of protein extract and 20-μl of sample buffer were
mixed in well-labelled Eppendorf tubes and a 40-μl sample solution
was formed. The 40-μl sample containing Eppendorf tubes
was placed in hot boiling water for 2-3 minutes and then allowed
to cool. Now, a 40-μl sample solution was loaded into the wells
present in the staking gel. The reference standard Bovine Serum
Albumin (BSA) was also loaded with each sample and assembly
was allowed to run at 130 Volts (35 mA) for 3-5 hours or until
the sample reached at the bottom of the gel. The gel was removed
form glass plates carefully and stained with Coomassie brilliant
blue R-250 for 24 hours. Then gel was replaced in de-staining
solution for 30 minutes to observe the bands visibility. Process for
replacing the de-staining solution was repeated if bands were not
clearly visible. The de-staining solution was the mixture of methanol,
acetic acid, and water with a ratio of 1:1:7 v/v.
When the gel was de-stained, and bands were observed on
the gel then photographs were taken. The bands were observed
manually, and their migration rate was recorded in a notebook for
relative mobility analysis. The relative mobility (Rf) can be calculated
by using the following formula:
Rf = distance migrated/dye front, length in gel
A 20-μl of protein extract and 20-μl of sample buffer were mixed in well-labelled Eppendorf tubes and a 40-μl sample solution was formed. The 40-μl sample containing Eppendorf tubes was placed in hot boiling water for 2-3 minutes and then allowed to cool. Now, a 40-μl sample solution was loaded into the wells present in the staking gel. The reference standard Bovine Serum Albumin (BSA) was also loaded with each sample and assembly was allowed to run at 130 Volts (35 mA) for 3-5 hours or until the sample reached at the bottom of the gel. The gel was removed form glass plates carefully and stained with Coomassie brilliant blue R-250 for 24 hours. Then gel was replaced in de-staining solution for 30 minutes to observe the bands visibility. Process for replacing the de-staining solution was repeated if bands were not clearly visible. The de-staining solution was the mixture of methanol, acetic acid, and water with a ratio of 1:1:7 v/v.
When the gel was de-stained, and bands were observed on the gel then photographs were taken. The bands were observed manually, and their migration rate was recorded in a notebook for relative mobility analysis. The relative mobility (Rf) can be calculated by using the following formula:
Rf = distance migrated/dye front, length in gel
Results and Discussion
Electrophoretic analysis using SDS-PAGE of the muscle
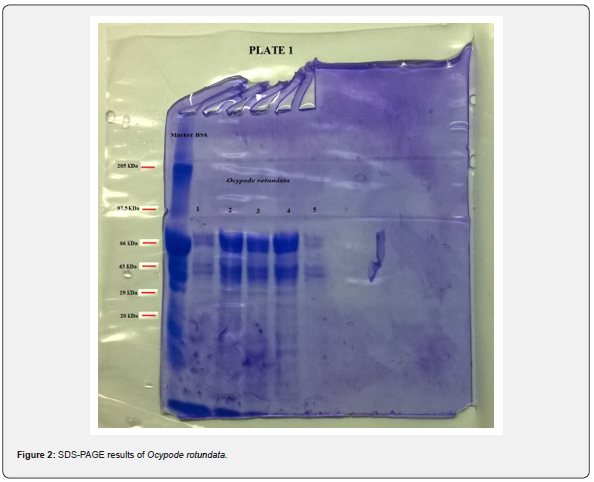
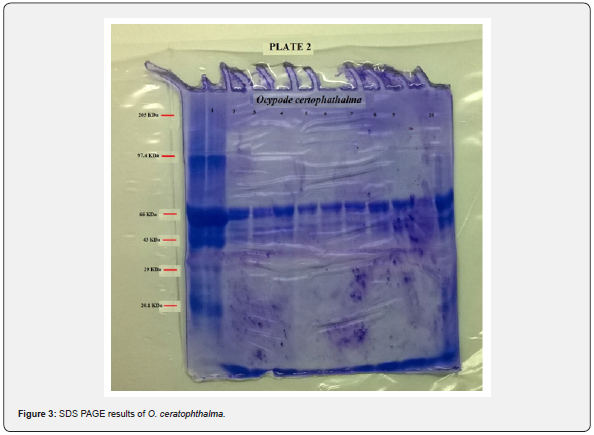
tissue was done. The present study revealed a total of 6 bands
(peptides) resolved in the muscle tissue of O. rotundata (Figure 2), whereas O. ceratophthalma revealed
a total of 8 bands (Figure 3). The four homologous proteins from band-3
to band-6 in both species were observed in the present investigation
with a small fractional difference. The band-5 in both species found a
similar size of BSA 66 kDa MW (Table 1). In the present study, several
thick protein bands with strong intensity can be clearly seen in O. rotundata and O. ceratophthalma based
on quantitative analysis with bands revealed 4 protein bands (band-1
to band-4) smaller than 66 kDa MW, whereas only one protein band
(band-6) in O. rotundata and 03 peptide bands (band-6 to band-8) in O. ceratophthalma found
larger in size than BSA (>66 kDa MW). The results of quantitative
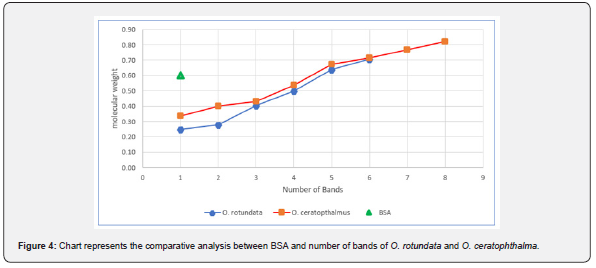
analysis of banding patterns using the SDS-PAGE can be seen in figure 4.
The smaller-sized protein bands were suspected to be myosin light chain
protein bands, sarcoplasmic calcium-binding proteins (SCBP),
tropomyosin, and arginine kinase ranging between 20 kDa to 40 kDa MW
comparing the present data with Arwani et al. [16]. Such results may
also be compared with allergen proteins in white shrimp as categorized
by the IUIS-International Union of Immunological Societies and
WHO-World Health Organisation, 2021. Other brachyuran crabs such as
Scylla serrata revealed thick with strong intensity bands ranging from
25 kDa to 65 kDa MW [17]. Arwani et al., [16] stated boiling protein
extract can greatly reduce the intensity and number of proteins up to
-36% in arginine kinase, -18% in myosin light chain, and increase the
tropomyosin protein intensity up to +528% in mud crab [18]. Kim et al.
[19] described that the reduced number of protein bands were likely to
be degraded into smaller molecular weight protein bands. It is also
possible that proteins that have a smaller resolution limit (less than
10% acrylamide) may travel faster and drop down into the buffer [16,20].
The muscle tissues collected from ghost crabs provided the
best results with clear resolution of protein bands using SDSPAGE.
The proteins present in muscle tissues can be used as marker
proteins to help in the various shellfish species identification
through the specific/unique protein bands present in each species’
muscle profile. The whole-body homogenates are less successful
and produce distinct proteins bands as compared to the
muscle tissues. Besides this, large number proteins may dilute the
essential proteins and prevent a precise quantitative analysis of
individual protein bands [21]. Dubey & Flynn [22] suggested that
protein resolution is controlled by percentage of acrylamide as
used in any study. Kitts et al. [21] recommended 12% acrylamide
gels for greater resolution of bands for muscle tissues.
The SDS-PAGE is the tool to separate protein extract
into specific
protein bands. These bands provide sources of identifying
differences between shellfish species [21]. Electrophoresis is a
simple technique for species identification, and it requires less
cost as compared to other species identification methods [23].
However, Scobbie and Maackie [24] argued that proteins get de-natured
due to boiling therefore, this method is not suitable for species
identification. Furthermore, due to the increasing demand
for shellfish species in the food market, it is necessary to
use reliable, sensitive methods to identify species to follow the
food and labeling regulations [21]. Therefore, An et al., [25] and
Taylor and Jones [26,27] suggested immunoassay methods such
as Enzyme-linked immunosorbent assays (ELISA) are useful for
shellfish species identification.
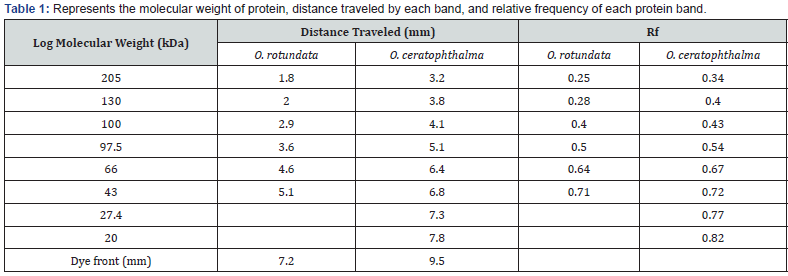
Electrophoretic analysis using SDS-PAGE of the muscle tissue was done. The present study revealed a total of 6 bands (peptides) resolved in the muscle tissue of O. rotundata (Figure 2), whereas O. ceratophthalma revealed a total of 8 bands (Figure 3). The four homologous proteins from band-3 to band-6 in both species were observed in the present investigation with a small fractional difference. The band-5 in both species found a similar size of BSA 66 kDa MW (Table 1). In the present study, several thick protein bands with strong intensity can be clearly seen in O. rotundata and O. ceratophthalma based on quantitative analysis with bands revealed 4 protein bands (band-1 to band-4) smaller than 66 kDa MW, whereas only one protein band (band-6) in O. rotundata and 03 peptide bands (band-6 to band-8) in O. ceratophthalma found larger in size than BSA (>66 kDa MW). The results of quantitative analysis of banding patterns using the SDS-PAGE can be seen in figure 4. The smaller-sized protein bands were suspected to be myosin light chain protein bands, sarcoplasmic calcium-binding proteins (SCBP), tropomyosin, and arginine kinase ranging between 20 kDa to 40 kDa MW comparing the present data with Arwani et al. [16]. Such results may also be compared with allergen proteins in white shrimp as categorized by the IUIS-International Union of Immunological Societies and WHO-World Health Organisation, 2021. Other brachyuran crabs such as Scylla serrata revealed thick with strong intensity bands ranging from 25 kDa to 65 kDa MW [17]. Arwani et al., [16] stated boiling protein extract can greatly reduce the intensity and number of proteins up to -36% in arginine kinase, -18% in myosin light chain, and increase the tropomyosin protein intensity up to +528% in mud crab [18]. Kim et al. [19] described that the reduced number of protein bands were likely to be degraded into smaller molecular weight protein bands. It is also possible that proteins that have a smaller resolution limit (less than 10% acrylamide) may travel faster and drop down into the buffer [16,20].

The muscle tissues collected from ghost crabs provided the best results with clear resolution of protein bands using SDSPAGE. The proteins present in muscle tissues can be used as marker proteins to help in the various shellfish species identification through the specific/unique protein bands present in each species’ muscle profile. The whole-body homogenates are less successful and produce distinct proteins bands as compared to the muscle tissues. Besides this, large number proteins may dilute the essential proteins and prevent a precise quantitative analysis of individual protein bands [21]. Dubey & Flynn [22] suggested that protein resolution is controlled by percentage of acrylamide as used in any study. Kitts et al. [21] recommended 12% acrylamide gels for greater resolution of bands for muscle tissues.
The SDS-PAGE is the tool to separate protein extract into specific protein bands. These bands provide sources of identifying differences between shellfish species [21]. Electrophoresis is a simple technique for species identification, and it requires less cost as compared to other species identification methods [23]. However, Scobbie and Maackie [24] argued that proteins get de-natured due to boiling therefore, this method is not suitable for species identification. Furthermore, due to the increasing demand for shellfish species in the food market, it is necessary to use reliable, sensitive methods to identify species to follow the food and labeling regulations [21]. Therefore, An et al., [25] and Taylor and Jones [26,27] suggested immunoassay methods such as Enzyme-linked immunosorbent assays (ELISA) are useful for shellfish species identification.



Conclusion
The use of SDS-PAGE in the current study is an effective method
for identifying species differences in ghost crabs. The SDS-PAGE is
also recommended for relative concentration, and molecular mass
estimation of proteins since 1970, as it provides a higher resolution
of protein bands [10,28-30]. A few soluble proteins may also
be used as markers for species identification. This methodology
provides necessary basic knowledge related to species-specific
protein bands that focus on the opportunities for biotechnology
as it is linked to immune-analytic techniques [31-39].
To Know More About Oceanography & Fisheries Open Access Journal Please click on:
https://juniperpublishers.com/ofoaj/index.php
For more Open Access Journals in Juniper Publishers please click on:
https://juniperpublishers.com/index.php
The use of SDS-PAGE in the current study is an effective method for identifying species differences in ghost crabs. The SDS-PAGE is also recommended for relative concentration, and molecular mass estimation of proteins since 1970, as it provides a higher resolution of protein bands [10,28-30]. A few soluble proteins may also be used as markers for species identification. This methodology provides necessary basic knowledge related to species-specific protein bands that focus on the opportunities for biotechnology as it is linked to immune-analytic techniques [31-39].
To Know More About Oceanography & Fisheries Open Access Journal Please click on:
https://juniperpublishers.com/ofoaj/index.php
For more Open Access Journals in Juniper Publishers please click on:
https://juniperpublishers.com/index.php

Comments
Post a Comment